25 mg, 50 mg, 75 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
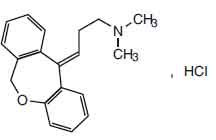
DOXEPIN CAPSULES B.P. (Doxepin Hydrochloride) is tricyclic antidepressant. Chemically, Doxepin Hydrochloride is (E)-3-(dibenzo[b,e]oxepin-11(6H)-ylidene)-N,N-dimethylpropan-1-amine hydrochloride. The molecular formula is C19H21NO,HCl and molecular weight is 315.8.
STRUCTURAL FORMULA :
Its structural formula is :
DOXEPIN CAPSULES B.P. 25 mg are almost white coloured powder filled in size 4 green/yellow capsules.
DOXEPIN CAPSULES B.P. 50 mg are almost white coloured powder filled in size 4 blue/white capsules.
DOXEPIN CAPSULES B.P. 75 mg are almost white coloured powder filled in size 4 maroon/pink capsules.
COMPOSITION :
Each hard gelatin capsule contains :
Doxepin HCl B.P.
equivalent to Doxepin 25 mg
Excipients q.s.
Approved colours used in empty capsule shells.
Each hard gelatin capsule contains :
Doxepin HCl B.P.
equivalent to Doxepin 50 mg
Excipients q.s.
Approved colours used in empty capsule shells.
Each hard gelatin capsule contains :
Doxepin HCl B.P.
equivalent to Doxepin 75 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Doxepin is a tricyclic antidepressant. The mechanism of action of doxepin is not definitely known. It is not a central nervous stimulant nor a monoamine oxidase inhibitor. The current hypothesis is that the clinical effects are due, at least in part, to influences on the adrenergic activity at the synapses so that deactivation of noradrenaline by re-uptake into the nerve terminals is prevented. In animal studies anticholinergic, antiserotonin and antihistamine effects on smooth muscle have been demonstrated. At higher than usual clinical doses, adrenaline response was potentiated in animals. This effect was not demonstrated in humans.
PHARMACOKINETICS :
Doxepin is well absorbed from the gastrointestinal tract. Approximately 55 - 87 % of orally administered doxepin undergoes first pass metabolism in the liver, forming the primary active metabolite, desmethyldoxepin.In healthy volunteers, a single oral dose of 75 mg resulted in peak plasma concentrations for doxepin ranging from 8.8 - 45.8 ng/ml (mean 26.1 ng/ml). Peak levels were reached between 2 and 4 hours (mean 2.9 hours) after administration. Peak levels for the primary metabolite desmethyldoxepin ranged from 4.8 - 14.5 ng/ml (mean 9.7 ng/ml) and were achieved between 2 and 10 hours after administration. The mean apparent volume of distribution for doxepin is approximately 20 L/kg. The protein binding for doxepin is approximately 76 %. In healthy volunteers, the plasma elimination half-life of doxepin ranged from 8 to 24 hours (mean 17 hours). The half-life of desmethyldoxepin ranged from 33 - 80 hours (mean 51 hours). Mean plasma clearance for doxepin is approximately 0.84 L/kg.hr. Paths of metabolism of doxepin include demethylation, N-oxidation, hydroxylation and glucuronide formation. Doxepin is excreted primarily in the urine, mainly as its metabolites, either free or in conjugate form.
INDICATIONS :
Doxepin is recommended for the treatment of :
1. Psychoneurotic patients with depression and/or anxiety.
2. Depression and/or anxiety associated with alcoholism (not to be taken concomitantly with alcohol).
3. Depression and/or anxiety associated with organic disease (the possibility of drug interaction should be considered if the patient is receiving other drugs concomitantly).
4. Psychotic depressive disorders with associated anxiety including involutional depression and manic-depressive disorders.
The target symptoms of psychoneurosis that respond particularly well to doxepin include anxiety, tension, depression, somatic symptoms and concerns, sleep disturbances, guilt, lack of energy, fear, apprehension and worry.Clinical experience has shown that doxepin is safe and well tolerated even in the elderly patient. Owing to lack of clinical experience in the paediatric population, doxepin is not recommended for use in children under 12 years of age.
Administration :
DOXEPIN CAPSULES B.P. is for oral administration.
Dosage :
The optimum oral dose depends on the severity of the condition and the individual patients response. The dose varies from 30 - 300 mg daily. Doses up to 100 mg daily may be given on a divided or once daily schedule. Should doses over 100 mg daily be required, they should be administered in three divided doses daily. 100 mg is the maximum dose recommended at any one time. This dose may be given at bedtime. For the majority of patients with moderate or severe symptoms, it is recommended that treatment commences with an initial dose of 75 mg daily. Many of these patients will respond satisfactorily at this dose level. For patients who do not, the dosage may be adjusted according to individual response. In more severely ill patients, it may be necessary to administer a dose of up to 300 mg, in three divided doses daily, to obtain a clinical response.
In patients where insomnia is a troublesome symptom, it is recommended that the total daily dose be divided so that a higher proportion is given for the evening dose; similarly, if drowsiness is experienced as a side effect of treatment, doxepin may be administered by this regimen, or the dosage may be reduced. It is often possible, having once obtained a satisfactory therapeutic response, to reduce the dose for maintenance therapy. The optimal antidepressant effect may not be evident for two to three weeks.
Adolescent Depression :
Not recommended for use in adolescent patients 13 - 18 years of age for the treatment of depression, unless under the supervision of a specialist.
Use in the Elderly :
In general, lower dosages are recommended. Where the presenting symptoms are mild in nature, it is advisable to initiate treatment at a dose of 10 - 50 mg daily. A satisfactory clinical response is obtained in many of these patients at a daily dose of 30 - 50 mg. The dosage may be adjusted according to the individual response.
Use in Hepatic Impairment :
Dosage reduction may be required in patients with hepatic impairment.
CONTRAINDICATIONS :
Doxepin is contraindicated for the treatment of depression in patients 12 years of age and under. Doxepin is contraindicated for the treatment of nocturnal enuresis. Hypersensitivity, mania, severe liver disease, lactation, glaucoma, tendency to urinary retention.
WARNINGS :
Clinical Worsening and Suicide Risk :
Patients of any age with Major Depressive Disorder may experience worsening of their depression and/or the emergence of suicidal ideation and behaviour (suicidality), whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Patients should be closely monitored, especially at the beginning of therapy or when the dose is changed, until such improvement occurs. There has been a long-standing concern that some antidepressants may have a role in the emergence of suicidality in some patients. The possible risk of increased suicidality in patients applies to all classes of antidepressant medicines, as available data are not adequate to exclude this risk for any antidepressant. Therefore, consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse or whose emergent suicidality is severe, abrupt in onset, or was not part of the patients presenting symptoms. Generally, when stopping an antidepressant, doses should be tapered rather than stopped abruptly.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and paediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality. Because of the possibility of co-morbidity between major depressive disorder and other psychiatric and non-psychiatric disorders, the same precautions observed when treating patients with major depressive disorder should be observed when treating patients with other psychiatric and non-psychiatric disorders.
Mania and Bipolar Disorder :
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with any antidepressant alone may increase the likelihood of a mixed/manic episode in patients at risk for bipolar disorder. Prior to initiating treatment with an antidepressant, patients should be adequately screened to determine if they are at risk for bipolar disorder. It should be noted that doxepin is not approved for use in treating bipolar depression. All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Screening Patients for Bipolar Disorder :
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that doxepin is not approved for use in treating bipolar depression. It should be noted that doxepin is not approved for use in treating any indications in the paediatric population. The once a day dosage regimen of doxepin in patients with intercurrent illness or patients taking other medications should be carefully adjusted. This is especially important in patients receiving other medications with anticholinergic effects.
PRECAUTIONS :
Clinical Worsening and Suicide Risk :
Patients, their families and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day to day basis, since changes may be abrupt. Such symptoms should be reported to the patients prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patients presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patients presenting symptoms.
Pregnancy : Pregnancy Category B
Doxepin crosses the placenta. Reproduction studies have been performed in rats, rabbits and monkeys and there was no evidence of harm to the animal foetus. The relevance to humans is not known. Since there is insufficient experience in pregnant women who have received this drug, its safety in pregnancy has not been established.
Nursing mothers :
Doxepin and its active metabolite, desmethyldoxepin, are excreted in breast milk. There has been a report of apnoea and drowsiness occurring in a nursing infant whose mother was taking doxepin. The use of doxepin is contraindicated during lactation.
INTERACTIONS AND INCOMPATIBILITIES :
Combined use with other antidepressants, alcohol or anti-anxiety agents should be undertaken with due recognition of the possibility of potentiation. It is known, for example, that monoamine oxidase inhibitors may potentiate other drug effects, therefore doxepin should not be given concurrently, or within two weeks of cessation of therapy, with monoamine oxidase inhibitors. Cimetidine has been reported to produce clinically significant fluctuations in steady-state serum concentrations of various tricyclic antidepressants. Anaesthetics given during tricyclic or tetracyclic antidepressant therapy may increase the risk of arrhythmias and hypotension. If surgery is necessary, the anaesthetist should be informed that a patient is being so treated. Use with caution in patients with a history of epilepsy.
Doxepin may decrease the antihypertensive effect of agents such as debrisoquine, bethanidine, guanethidine and possibly clonidine. It usually requires daily doses of doxepin in excess of 150 mg before any effect on the action of guanethidine is seen. It would be advisable to review all antihypertensive therapy during treatment with tricyclic antidepressants. Doxepin should not be given with sympathomimetic agents, such as ephedrine, isoprenaline, noradrenaline, phenylephrine and phenylpropanolamine. Barbiturates may increase the rate of metabolism of doxepin. The dose of thyroid hormone medication may need reducing if doxepin is being given concurrently. Tolazamide : A case of severe hypoglycaemia 11 days after the addition of doxepin (75 mg/day) has been reported in a non-insulin dependent diabetic patient maintained on tolazamide (1 g/day).
SIDE EFFECTS :
Note : Some of the side-effects noted below have not been specifically reported with doxepin. However, due to the close pharmacological similarities amongst the tricyclics, the reactions should be considered when prescribing doxepin.
Anticholingeic Effects : Dry mouth, blurred vision, constipation and urinary retention have been reported. If they do not subside with continued therapy, or if they become severe, it may be necessary to reduce the dosage.
Central Nervous System Effects : Drowsiness is the most commonly noticed side-effect. This tends to disappear as therapy is continued. Other infrequently reported CNS side-effects are confusion, disorientation, agitation, hallucinations, numbness, paraesthesiae, ataxia, extrapyramidal symptoms, tardive dyskinesia, tremor and convulsions. Psychotic manifestations, including mania and paranoid delusions may be exacerbated during treatment with tricyclic antidepressants.
Cardiovascular : Although doxepin carries less risk than other tricyclic antidepressants, caution should be observed in the treatment of patients with heart block or cardiac arrhythmias. Cardiovascular effects including hypotension, hypertension and tachycardia have been reported occasionally.
Allergic : Skin rash, facial oedema, photosensitisation and pruritus have occasionally occurred.
Haematological : Eosinophilia has been reported in a few patients. There have been occasional reports of bone marrow depression manifesting as agranulocytosis, leukcopaenia, thrombocytopaenia and purpura.
Gastro-intestinal : Nausea, vomiting, indigestion, taste disturbances, diarrhoea, anorexia and aphthous stomatitis have been reported.
Endocrine : Raised or lowered libido, testicular swelling, gynaecomastia, enlargement of breasts and galactorrhoea in the female, raising or lowering of blood sugar levels and inappropriate antidiuretic hormone secretion, have been reported following the administration of tricyclics.
Other : Dizziness, tinnitus, weight gain, sweating, chills, fatigue, weakness, flushing, jaundice, alopecia, headache, exacerbation of asthma and hyperpyrexia (in association with chlorpromazine) have been occasionally observed.
INFORMATION FOR PATIENTS :
Patients and their families should be alerted about the need to monitor for the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, impulsivity, akathisia, hypomania, mania, worsening of depression, and suicidal ideation, especially early during antidepressant treatment. Such symptoms should be reported to the patients doctor, especially if they are severe, abrupt in onset, or were not part of the patients presenting symptoms. The patient has the right to treatment meeting appropriate ethical and professional standards, and the patient needs to be fully informed with frank discussion of risk/benefit issues relating to the medicines efficacy and safety when used in the treatment regimen proposed. The once-a-day dosage regimen of doxepin in patients with intercurrent illness or patients taking other medications should be carefully adjusted. This is especially important in patients receiving other medications with anticholinergic effects.
The use of doxepin on a once-a-day dosage regimen in geriatric patients should be adjusted carefully on the basis of the patients condition. The elderly are particularly liable to experience toxic effects, especially agitation, confusion and postural hypotension. The initial dose should be increased with caution under close supervision. Half the normal maintenance dose may be sufficient to produce a satisfactory clinical response. Patients should be warned that drowsiness may occur with the use of doxepin. Patients should also be cautioned that their response to alcohol may be potentiated. Use with caution in patients with severe cardiovascular disease, including patients with heart block, cardiac arrhythmia and those who have experienced a recent myocardial infarction. Use with caution in patients with hepatic and/or renal impairment. Use with caution in patients with a history of epilepsy.
Driving/Use of Machinery :
Since drowsiness may occur with the use of doxepin, patients should be warned of the possibility and cautioned against driving a car or operating machinery while taking this drug.
OVERDOSAGE :
Signs and symptoms :
Mild : Drowsiness, stupor, blurred vision, excessive dryness of mouth.
Severe : Respiratory depression, hypotension, coma, convulsions, cardiac arrhythmias and tachycardias.
Also : urinary retention (bladder atony), decreased gastrointestinal motility (paralytic ileus), hyperthermia (or hypothermia), hypertension, dilated pupils, hyperactive reflexes.
TREATMENT OF OVERDOSAGE :
Mild :
Observation and supportive therapy is all that is usually necessary.
Severe :
Medical management of severe doxepin overdosage consists of aggressive supportive therapy. If the patient is conscious, gastric lavage, with appropriate precautions to prevent pulmonary aspiration, should be performed even though doxepin is rapidly absorbed. The use of activated charcoal has been recommended, as has been continuous gastric lavage with saline for 24 hours or more. An adequate airway should be established in comatose patients and assisted ventilation used if necessary. ECG monitoring may be required for several days, since relapse after apparent recovery has been reported. Arrhythmias should be treated with the appropriate anti-arrhythmic agent. It has been reported that many of the cardiovascular and CNS symptoms of tricyclic antidepressant poisoning in adults may be reversed by the slow intravenous administration of 1mg to 3mg of physostigmine salicylate. Because physostigmine is rapidly metabolised, the dosage should be repeated as required. Convulsions may respond to standard anticonvulsant therapy. However, barbiturates may potentiate any respiratory depression. Dialysis and forced diuresis generally are not of value in the management of overdosage due to high tissue and protein binding of doxepin.
STORAGE :
Store below 250C (770F), protected from moisture and light. Do not refrigerate.
SHELF LIFE :
36 months from the date of manufacture.
PRESENTATION :
DOXEPIN CAPSULES B.P. contains doxepin B.P. 25 mg/50 mg/75 mg.
100 Capsules in HDPE Bottle.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular