250 mg, 500 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
IMIPENEM AND CILASTATIN FOR INJECTION USP is a broad spectrum beta-lactam antibiotic. It consists of two components : (1) Imipenem, the first new class of beta-lactam antibiotics, the thienamycins : and (2) Cilastatin sodium, a specific enzyme that blocks the metabolism of Imipenem in the kidney, and substantially increases the concentration of intact imipenem in the urinary tract. Imipenem and Cilastatin sodium are present in IMIPENEM AND CILASTATIN FOR INJECTION USP in a 1:1 ratio by weight.
Chemically, Imipenem is (5R, 6S )-3-[[2-(Formimidoylamino)ethyl]thio]-6-[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate. The molecular formula is C12H17N3O4S ·H2O and the molecular weight is 317.36. Chemically, Cilastatin Sodium is Sodium (Z)-7-[[(R)-2-Amino-2-carboxyethyl]thio]-2-[(S)-2,2-dimethylcyclo propanecarboxamido]-2 heptenoate.The molecular formula is C16H25N2NaO5S and molecular weight is 380.44.
STRUCTURAL FORMULA :
Its structural formula is :
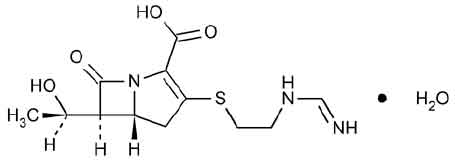
IMIPENEM AND CILASTATIN FOR INJECTION USP is a sterile white to light yellow powder filled in vial of suitable size.
COMPOSITION :
Each vial contains :
Each Imipenem USP
equivalent to anhydrous Imipenem 250 mg
Sterile Cilastatin Sodium USP
equivalent to Cilastatin 250 mg
Sterile Sodium Bicarbonate USP
(added as buffer)
Each vial contains :
Sterile Imipenem USP
equivalent to anhydrous Imipenem 500 mg
Sterile Cilastatin Sodium USP
equivalent to Cilastatin 500 mg
Sterile Sodium Bicarbonate USP
(added as buffer)
ACTIONS :
Cilastatin is a competitive, reversible and specific inhibitor of dehydropeptidase-I enzyme, the renal enzyme which metabolises and inactivates imipenem. It is devoid of intrinsic antibacterial activity and does not affect the antibacterial activity of imipenem. Imipenem is a beta-lactam antibiotic belonging to the thienamycin group. It is a potent inhibitor of bacterial cell wall synthesis and is bactericidal against a broad spectrum of pathogens - Gram-positive and Gram-negative, aerobic and anaerobic. IMIPENEM AND CILASTATIN FOR INJECTION USP shares with the new cephalosporins and penicillins a broad spectrum of activity against Gram-negative species, but is unique in retaining the high potency against Gram-positive species, previously associated only with earlier narrow-spectrum beta-lactam antibiotics. The spectrum of activity of IMIPENEM AND CILASTATIN FOR INJECTION USP includes Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis and Bacteroides fragilis, a diverse group of problem pathogens commonly resistant to other antibiotics. IMIPENEM AND CILASTATIN FOR INJECTION USP is resistant to degradation by bacterial beta-lactamases, which makes it active against a high percentage of organisms such as Pseudomonas aeruginosa, Serratia spp., and Enterobacter spp. which are inherently resistant to most beta-lactam antibiotics. The antibacterial spectrum of IMIPENEM AND CILASTATIN FOR INJECTION USP is broader than that of any other antibiotic studied, and includes virtually all clinically significant pathogens. Organisms against which IMIPENEM AND CILASTATIN FOR INJECTION USP is usually active in vitro include :
Susceptibility Testing
Clinical and Laboratory Standards Institute (CLSI) (Formerly NCCLS) - Methods of Susceptibility Testing
SUSCEPTIBILITY TESTS
When available, the results of in vitro susceptibility tests should be provided to the physician as periodic reports which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques :
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardised procedure. Standardised procedures are based on a dilution method (broth or agar) or equivalent with standardised inoculum concentrations and standardised concentrations of imipenem powder. The MIC values should be interpreted according to criteria provided in Table 1.
Diffusion Techniques :
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardised procedure requires the use of standardised inoculum concentrations. This procedure uses paper disks impregnated with 10 mcg imipenem to test the susceptibility of microorganisms to imipenem. The disk diffusion interpretive criteria are provided in Table 1.
Anaerobic Techniques :
For anaerobic bacteria, susceptibility to imipenem as MICs can be determined by a standardised test method. The MIC values obtained should be interpreted according to the criteria provided in Table 1.
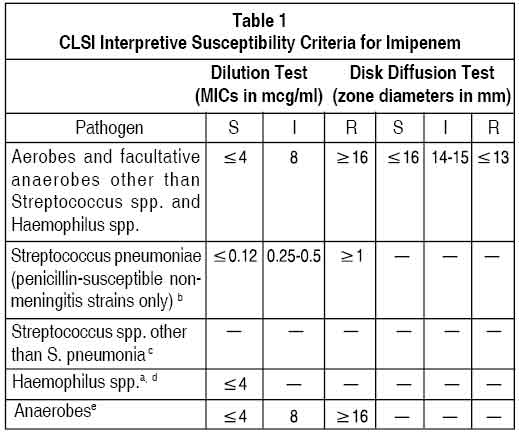
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable ; other therapy should be selected.
Quality Control :
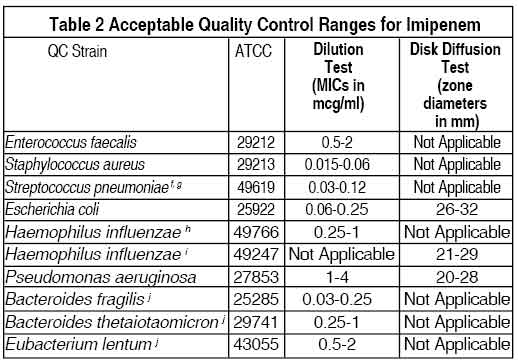
Standardised susceptibility test procedures require the use of quality control microorganisms to control the technical aspects of the test procedures. Standard imipenem powder should provide the following range of values noted in Table 2. Quality control microorganisms are specific strains of organisms with intrinsic biological properties. QC strains are very stable strains which will give a standard and repeatable susceptibility pattern. The specific strains used for microbiological quality control are not clinically significant.
PHARMACOKINETICS :
Intravenous infusion of imipenem-cilastatin over 20 minutes results in peak plasma levels of imipenem antimicrobial activity that range from 14 to 24 μg/ml for the 250 mg dose, from 21 to 58 g/ml for the 500 mg dose. At these doses, plasma levels of imipenem antimicrobial activity decline to below 1 μg/ml or less in 4 to 6 hours. The plasma half-life of each component is approximately 1 hour. The binding of imipenem to human serum proteins is approximately 20 % and that of cilastatin is approximately 40 %. Approximately 70 % of the administered imipenem is recovered in the urine within 10 hours after which no further urinary excretion is detectable. Urine concentrations of imipenem in excess of 10 μg/ml can be maintained for up to 8 hours with imipenem-cilastatin at the 500 mg dose. Approximately 70 % of the cilastatin sodium dose is recovered in the urine within 10 hours of administration
IMIPENEM-CILASTATIN.
No accumulation of imipenem/cilastatin in plasma or urine is observed with regimens administered as frequently as every 6 hours in patients with normal renal function. In healthy elderly volunteers (65 to 75 years of age with normal renal function for their age), the pharmacokinetics of a single dose of imipenem 500 mg and cilastatin 500 mg administered intravenously over 20 minutes are consistent with those expected in subjects with slight renal impairment for which no dosage alteration is considered necessary. The mean plasma half-lives of imipenem and cilastatin are 91 ± 7.0 minutes and 69 ± 15 minutes, respectively. Multiple dosing has no effect on the pharmacokinetics of either imipenem or cilastatin, and no accumulation of imipenem/cilastatin is observed. Imipenem, when administered alone, is metabolized in the kidneys by dehydropeptidase I resulting in relatively low levels in urine. Cilastatin sodium, an inhibitor of this enzyme , effectively prevents renal metabolism of imipenem so that when imipenem and cilastatin sodium are given concomitantly, fully adequate antibacterial levels of imipenem are achieved in the urine.
INDICATIONS :
IMIPENEM AND CILASTATIN FOR INJECTION USP is indicated for the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below :
1.Lower respiratory tract infections.
2. Urinary tract infections
3. Intra-abdominal infections.
4.Gynaecologic infections.
5.Bacterial septicemia.
6. Bone and joint infections.
7. Skin and skin structure infections.
8. Endocarditis.
9. Polymicrobic infections including those in which S. pneumoniae (pneumonia, septicemia), S. pyogenes (skin and skin structure), or nonpenicillinase-producing S. aureus is one of the causative organisms. However, monobacterial infections due to these organisms are usually treated with narrower spectrum antibiotics, such as penicillin G.
Administration :
For I.V.use only.
Dosage :
Adults
The dosage recommendations for IMIPENEM AND CILASTATIN FOR INJECTION USP represent the quantity of imipenem to be administered. An equivalent amount of cilastatin is also present in the solution. Each 125 mg, 250 mg, or 500 mg dose should be given by intravenous administration over 20 to 30 minutes. Each 750 mg or 1000 mg dose should be infused over 40 to 60 minutes. In patients who develop nausea during the infusion, the rate of infusion may be slowed.The total daily dosage for IMIPENEM AND CILASTATIN FOR INJECTION USP should be based on the type or severity of infection and given in equally divided doses based on consideration of degree of susceptibility of the pathogen(s), renal function, and body weight. Adult patients with impaired renal function, as judged by creatinine clearance ≥70 ml/min/ 1.73 m2, require adjustment of dosage as described in the succeeding section of these guidelines. Intravenous Dosage Schedule for Adults with Normal Renal Function and Body Weight ≥70 kg. Doses cited in Table I are based on a patient with normal renal function and a body weight of 70 kg. These doses should be used for a patient with a creatinine clearance of ≥71 ml/min/1.73 m2 and a body weight of ≥70 kg. A reduction in dose must be made for a patient with a creatinine clearance of ≥70 ml/min/1.73 m2 and/or a body weight less than 70 kg. Dosage regimens in column A of Table I are recommended for infections caused by fully susceptible organisms which represent the majority of pathogenic species. Dosage regimens in column B of Table I are recommended for infections caused by organisms with moderate susceptibility to imipenem, primarily some strains of P. aeruginosa.
Paediatric Patients
For paediatric patients ≥3 months of age, the recommended dose for non-CNS infections is 15–25 mg/kg/dose administered every six hours. Based on studies in adults, the maximum daily dose for treatment of infections with fully susceptible organisms is 2.0 g per day, and of infections with moderately susceptible organisms (primarily some strains of P. aeruginosa) is 4.0 g/day. Higher doses (up to 90 mg/kg/day in older children) have been used in patients with cystic fibrosis. For paediatric patients ≤3 months of age (weighing ≥1,
500 gms), the following dosage schedule is recommended for non-CNS infections :
<1 wk of age : 25 mg/kg every 12 hrs
1-4 wks of age : 25 mg/kg every 8 hrs
4 wks-3 months. of age : 25 mg/kg every 6 hrs.
Doses less than or equal to 500 mg should be given by intravenous infusion over 15 to 30 minutes. Doses greater than 500 mg should be given by intravenous infusion over 40 to 60 minutes. IMIPENEM AND CILASTATIN FOR INJECTION USP is not recommended in paediatric patients with CNS infections because of the risk of seizures. IMIPENEM AND CILASTATIN FOR INJECTION USP is not recommended in paediatric patients <30 kg with impaired renal function, as no data are available.
Directions for Use
Vials
Contents of the vials must be suspended and transferred to 100 ml of an appropriate infusion solution. A suggested procedure is to add approximately 10 ml from the appropriate infusion solution to the vial. Shake well and transfer the resulting suspension to the infusion solution container. Benzyl alcohol as a preservative has been associated with toxicity in neonates. While toxicity has not been demonstrated in paediatric patients greater than three months of age, small paediatric patients in this age range may also be at risk for benzyl alcohol toxicity. Therefore, diluents containing benzyl alcohol should not be used when IMIPENEM AND CILASTATIN FOR INJECTION USP is constituted for administration to paediatric patients in this age range. Repeat with an additional 10 ml of infusion solution to ensure complete transfer of vial contents to the infusion solution. The resulting mixture should be agitated until clear.
CONTRAINDICATIONS :
IMIPENEM AND CILASTATIN FOR INJECTION USP is contraindicated in patients who have shown hypersensitivity to any component of this product.
WARNINGS :
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS RECEIVING THERAPY WITH BETA-LACTAMS. THESE REACTIONS ARE MORE APT TO OCCUR IN PERSONS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF PATIENTS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE HYPERSENSITIVITY REACTIONS WHEN TREATED WITH ANOTHER BETA-LACTAM. BEFORE INITIATING THERAPY WITH IMIPENEM AND CILASTATIN FOR INJECTION USP, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OTHER BETA LACTAMS, AND OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, IMIPENEM AND CILASTATIN FOR INJECTION USP SHOULD BE DISCONTINUED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, MAY ALSO BE ADMINISTERED AS INDICATED.
Seizure Potential
Seizures and other CNS adverse experiences, such as confusional states and myoclonic activity, have been reported during treatment with IMIPENEM AND CILASTATIN FOR INJECTION USP. Case reports in the literature have shown that co-administration of carbapenems, including imipenem, to patients receiving valproic acid or divalproex sodium results in a reduction in valproic acid concentrations. The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures. Increasing the dose of valproic acid or divalproex sodium may not be sufficient to overcome this interaction. The concomitant use of imipenem and valproic acid/divalproex sodium is generally not recommended. Anti-bacterials other than carbapenems should be considered to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium. If administration of IMIPENEM AND CILASTATIN FOR INJECTION USP is necessary, supplemental anti-convulsant therapy should be considered. Clostridium difficile associated diarrhoea (CDAD) has been reported with use of nearly all antibacterial agents, including IMIPENEM AND CILASTATIN FOR INJECTION USP, and may range in severity from mild diarrhoea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD.
Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS :
Cross-sensitivity and/ or related problems :
Patients allergic to other beta-lactam antibacterials (e.g., Penicillins, cephalosporins) may be allergic to imipenem also.
Although imipenem has been administered without incident to some patients with rash-type peniciilin allergy,caution is recommended when imipenem is administered to patients with a history of penicillin anaphylaxis because of cross-reactivity.
Carcinogenicity/Mutagenicity :
Gene toxicity studies such as the V mammalian assay, Ames test, unscheduled DNA synthesis assay, and in vivo mouse cytogenicity test have shown no evidence of generic damage with imipenem and cilastatin combination.
Pregnancy : Category C
IMIPENEM AND CILASTATIN FOR INJECTION USP should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and foetus.
Nursing mothers :
It is not known whether imipenem-ciclastatin sodium is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IMIPENEM AND CILASTATIN FOR INJECTION USP is administered to a nursing women.
Paediatric Use :
The half-life of imipenem in neonates is longer (1.7 to 2.4 hours) than that in adults with normal renal function (approximately 1 hour). The half-life in older paediatric patients (2 to 12 years of age) is 1 to 1.2 hours. The half-life of cilastatin in neonates is longer (3.8 to 8.4 hours) than that in adults with normal renal function (approximately 1 hour). Appropriate studies have not been performed in children upto 12 years of age.
Effects on ability to drive and use machines :
There are no specific data ;however, some of the CNS side-effects, such as dizziness, psychic disturbances, confusion and seizures, may affect the ability to drive or operate machinery.
INTERACTIONS AND INCOMPATIBILITIES :
Interactions :
General seizures have been reported in patients who received ganciclovir and IMIPENEM AND CILASTATIN FOR INJECTION USP. These drugs should not be used concomitantly unless the potential benefit outweighs the risk. Decreases in valproic acid levels that may fall below the therapeutic range have been reported when valproic acid was co-administered with carbapenem agents. The lowered valproic acid levels can lead to inadequate seizure control; therefore, concomitant use of imipenem and valproic acid/sodium valproate is not recommended and alternative antibacterial or anti-convulsant therapies should be considered. Concomitant probenecid has been shown to double the plasma level and half-life of cilastatin, but with no effect on its urinary recovery. Concomitant probenecid showed only minimal increases in plasma level and half-life of imipenem, with urinary recovery of active imipenem decreased to approximately 60 % of the administered dose.
Incompatibilities :
IMIPENEM AND CILASTATIN FOR INJECTION USP is chemically incompatible with lactate and should not be reconstituted with diluents containing lactate.
IMIPENEM AND CILASTATIN FOR INJECTION USP can, however, be administered into an IV tubing through which a lactate solution is being infused.
IMIPENEM AND CILASTATIN FOR INJECTION USP should not be mixed or physically added to other antibiotics.
SIDE EFFECTS :
IMIPENEM AND CILASTATIN FOR INJECTION USP is generally well tolerated. Side effects rarely require cessation of therapy and are generally mild and transient ; serious side effects are rare.
Local reactions : Erythema, local pain and induration, thrombophlebitis.
Allergic : Rash, pruritus, urticaria, erythema multiform , Stevens-Johnson syndrome, angiooedema, toxic epidermal necrolysis (rarely), exfoliative dermatitis, (rarely) candidiasis, fever including drug fever, anaphylactic reactions.
Gastro-intestinal : Nausea, vomiting, diarrhoea, staining of teeth and/or tongue. Pseudomembranous colitis has been reported.
Blood : Eosinophilia, leucopaenia, neutropaenia including agranulocytosis, thrombocytopaenia, thrombocytosis, decreased haemoglobin and prolonged prothrombin time. A positive direct Coombs test may develop.
Liver function : Mild increases in serum transaminases, bilirubin and/or serum alkaline phosphatase, hepatitis rarely have been reported.
Renal function : Oliguria/anuria, polyuria, acute renal failure (rarely). The role of IMIPENEM AND CILASTATIN FOR INJECTION USP in changes in renal function is difficult to assess, since factors predisposing to pre-renal uraemia or to impaired renal function usually have been present. Elevated serum creatinine and blood urea have been seen. A harmless urine discoloration, not to be confused with haematuria, has been seen in children.
Central nervous system : Myoclonic activity, psychic disturbances including hallucinations, paraesthesia, confusional states or convulsions have been reported.
Granulocytopaenic patients : Drug-related nausea and/or vomiting appear to occur more frequently in granulocytopaenic patients than in non-granulocytopaenic patients treated with IMIPENEM AND CILASTATIN FOR INJECTION USP.
Special senses : Hearing loss, taste perversion. Other reported reactions with an unknown causal relationship
Gastro-intestinal : Haemorrhagic colitis, gastro-enteritis, abdominal pain, glossitis, tongue papillar hypertrophy, heartburn, pharyngeal pain, increased salivation.
Central nervous system : Dizziness, somnolence, encephalopathy, vertigo, headache.
Special senses : Tinnitus.
Respiratory : Chest discomfort, dyspnoea, hyperventilation, thoracic spine pain.
Cardiovascular : Hypotension, palpitations, tachycardia.
Skin : Flushing, cyanosis, hyperhidrosis, skin texture changes, pruritus vulvae.
Body as a whole : Polyarthralgia, asthaenia/weakness.
Blood : Haemolytic anaemia, pancytopaenia, bone marrow depression
OVERDOSAGE :
Symptoms of overdosage include neuromuscular hyper sensitivity, seizures.
TREATMENT OF OVERDOSAGE :
Haemodialysis may be helpful to aid in the removal of the drug from the blood ; otherwise most treatment is supportive or symptom directed.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C(86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
IMIPENEM AND CILASTATIN FOR INJECTION USP contains Sterile Imipenem USP equivalent to anhydrous Imipenem 250 mg and Sterile Cilastatin Sodium USP equivalent to Cilastatin 250 mg.
Single vial pack.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular