1 gm
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
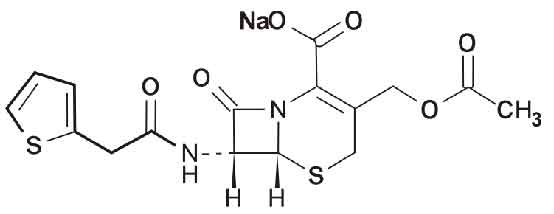
Cephalothin sodium is a first-generation cephalosporin antibacterial that has been used in the treatment of infections due to susceptible bacteria, particularly staphylococci. Chemically, Cephalothin sodium is 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(acetyloxy)methyl]-8-oxo-7-[(2-thienylacetyl)amino]-, monosodium salt, (6R-trans)-. The molecular formula is C16H15N2NaO6S2 and molecular weight is 418.42.
STRUCTURAL FORMULA :
Its structural formula is :
CEPHALOTHIN FOR INJECTION USP is a sterile off white powder filled in a flint tubular vial of suitable size.
COMPOSITION :
Each vial contains :
Sterile Cephalothin Sodium USP
equivalent to Cephalothin 1 gm
ACTIONS :
Cephalothin is a beta-lactam antibacterial. It is bactericidal and acts by inhibiting synthesis of the bacterial cell wall. It is most active against gram-positive cocci, and has moderate activity against some gram-negative bacilli. It exerts its killing action on growing and dividing bacteria by inhibiting bacterial cell-wall synthesis, although the mechanisms involved are still not precisely understood. Bacterial cell walls are held rigid and protected against osmotic rupture by peptidoglycan. Cephalothin inhibits the final cross-linking stage of peptidoglycan production by binding to and inactivating transpeptidases, penicillin-binding proteins on the inner surface of the bacterial cell membrane.
Spectrum of activity :
Sensitive gram-positive cocci include both penicillinase- and non-penicillinase-producing staphylococii, although meticillin-resistant staphylococci are resistant; most streptococci and some gram-positive anaerobes are also susceptible. Cephalothin is usually inactive against Listeria monocytogenes. Among gram-negative bacteria cephalothin has activity against some Enterobacteriaceae including strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Salmonella, and Shigella spp., but not against Enterobacter, indole-positive Proteus, or Serratia spp. It is also active against Moraxella catarrhalis (Branhamella catarrhalis) and Neisseria spp., though Haemophilus influenzae is moderately resistant. Bacteroides fragilis and Pseudomonas aeruginosa are not sensitive and neither are mycobacteria, mycoplasma and fungi.
Resistance :
Resistance of bacteria to cephalothin may be due to several mechanisms : the drug may be prevented from reaching its site of action, for example in some gram-negative organisms the cell wall may be a potential barrier; the target penicillin-binding proteins may be altered so that cephalothin cannot bind with these proteins; or, most importantly, the organism may produce beta-lactamases (cephalosporinases). Cephalothin is relatively resistant to hydrolysis by staphylococcal beta-lactamase, but is inactivated by a variety of beta-lactamases produced by gram-negative organisms; resistance of gram-negative organisms often depends on more than one factor. Resistance can be chromosomally or plasmid-mediated and may sometimes be inducible by cephalosporins. Some cross-resistance may occur between cephalothin and the penicillinase-resistant penicillins.
PHARMACOKINETICS :
Absorption :
Cephalothin is poorly absorbed from the gastrointestinal tract. After intramuscular injection peak plasma concentrations of about 10 and 20 mcg/ml are achieved within 30 minutes of doses of 0.5 and 1 g, respectively. A concentration of 30 mcg/ml has been reported 15 minutes after the intravenous injection of a 1 g dose; a range of 14 to 20 mcg/ml has been achieved by the continuous intravenous infusion of 500 mg/hour.
Distribution :
Cephalothin is widely distributed in body tissues and fluids except the brain and CSF where the concentrations achieved are low and unpredictable. It crosses the placenta and low concentrations have been detected in breast milk.The plasma half-life varies from about 30 to 50 minutes, but may be longer in patients with renal impairment, especially that of the metabolite. About 70 % of cephalothin is bound to plasma proteins.
Metabolism / Elimination :
Approximately 20 to 30 % of cephalothin is rapidly deacetylated in the liver and about 60 to 70 % of a dose is excreted in the urine by the renal tubules within 6 hours as cephalothin and the less active metabolite, desacetylcephalothin. High urine concentrations of 0.8 and 2.5 mg/ml have been observed following intramuscular doses of 0.5 and 1 g, respectively. Probenecid blocks the renal excretion of cephalothin. A very small amount is excreted in bile.
INDICATIONS :
Treatment of infections when caused by susceptible strains in respiratory, genitourinary, gastrointestinal, skin and soft tissue, bone and joint infections; septicaemia; treatment of susceptible gram-positive bacilli and cocci (never enterococcus); some gram-negative bacilli including E. coli, Proteus, and Klebsiella may be susceptible.
Administration :
Cephalothin is given as the sodium salt by slow intravenous injection over 3 to 5 minutes or by intermittent or continuous infusion. There may be pain at the injection site following intramuscular use, and thrombophlebitis has occurred following intravenous infusion of cephalosporins.
Dosage :
Usual Adult and adolescent dose :
- For Furunculosis, with cellulitis; Pneumonia, uncomplicated; Urinary tract infection : Intramuscular or intravenous, 500 mg every 6 hours.
- For Perioperative prophylaxis : Intravenous, 2 g one-half to one hour prior to the start of surgery; 2 g during surgery; and 2 g every 6 hours following surgery for up to 48 hours.
- For all other infection : Intramuscular or intravenous, 500 mg to 2 g every 4 to 6 hours.
Adult prescribing limits : 12 g per day.
Usual Paediatric dose :
For bacterial infections : Intramuscular or intravenous, 13.3 to 26.6 mg per kg of body weight every 4 hours; or 20 to 40 mg per kg of body weight every 6 hours.
Usual Geriatric dose : See Usual Adult and adolescent dose.
Reconstitution :
To prepare initial dilution for intramuscular use, 4.5 ml of sterile water for injection should be added to each 1 gram vial. To prepare initial dilution for intravenous use, 10 ml of sterile water for injection, 5 % dextrose injection, or 0.9 % sodium chloride injection should be added to each 1 g vial. For direct or intermittent use, the resulting solution should be administered over a 3 to 5 minute period. For continuous infusion, the resulting solution should be further diluted in suitable fluids.
Stability :
After reconstitution, solutions retain their potency for 8 hours at room temperature or for 72 hours if refrigerated. Solutions reconstituted with bacteriostatic diluent and used for intramuscular administration retain their potency for up to 7 days when refrigerated.
Concentrated solutions will darken in colour, especially at room temperature. However, slight discoloration does not affect potency.
CONTRAINDICATIONS :
Cephalothin is contraindicated in patients who have a history of hypersensitivity reactions to penicillins or cephalosporins.
WARNINGS AND PRECAUTIONS :
Care is necessary in patients with a history of allergy. Cephalothin should be given with caution to patients with renal impairment; dosage reduction may be necessary. Renal and haematological status should be monitored especially during prolonged and high-dose therapy.
Pregnancy : Category B
Nursing mothers :
Cephalothin is excreted in breast milk and may cause the infant to develop diarrhoea, candidiasis (yeast infection), or an allergic reaction. Caution should therefore be exercised by balancing the potential benefits of treatment against any possible hazard.
INTERACTIONS AND INCOMPATIBILITIES :
Drug-Drug Interaction :
- The use of nephrotoxic drugs such as the aminoglycosides gentamicin and tobramycin may increase the risk of kidney damage with cephalothin.
- There is also some evidence for enhanced nephrotoxicity with the loop diuretic furosemide.
- The renal excretion of cephalothin is inhibited by probenecid.
- There may be antagonism between cephalothin and bacteriostatic antibacterials.
Drug-Laboratory Interaction :
- Cephalothin may interfere with the Jaffé method of measuring creatinine concentrations and may produce falsely high values; this should be borne in mind when measuring renal function.
- Positive results to the direct Coombs’ test have been found during treatment with cephalothin and these can interfere with blood cross-matching.
- The urine of patients being treated with cephalothin may give false-positive reactions for glucose using copper-reduction reactions.
Incompatibilities :
The admixture of cephalothin and aminoglycosides may result in substantial mutual inactivation. If they are administered concurrently, they should be administered in separate sites. Do not mix them in the same intravenous bag or bottle.
SIDE EFFECTS :
The most common are hypersensitivity reactions, including skin rashes, urticaria, eosinophilia, fever, reactions resembling serum sickness, and anaphylaxis. Neutropaenia and thrombocytopaenia have occasionally been reported. Nephrotoxicity has been reported with cephalothin although it is less toxic. Acute renal tubular necrosis has followed excessive dosage and has also been associated with its use in older patients or those with pre-existing renal impairment, or when used with nephrotoxic drugs such as aminoglycosides. Acute interstitial nephritis is also a possibility as a manifestation of hypersensitivity. Convulsions and other signs of CNS toxicity have been associated with high doses, especially in patients with severe renal impairment. There may be a positive response to the Coombs’ test although haemolytic anaemia rarely occurs.
OVERDOSAGE :
Symptoms of overdose include neuromuscular hypersensitivity, convulsions especially with renal insufficiency.
TREATMENT OF OVERDOSAGE :
Haemodialysis may be helpful to aid in the removal of the drug from the blood, otherwise most treatment is supportive or symptom-directed.
PHARMACEUTICAL PRECAUTIONS :
On reconstitution, parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate. The reconstituted solution should be used immediately after preparation.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CEPHALOTHIN FOR INJECTION USP is supplied as :

Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular