2.5 mg, 5 mg, 10 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
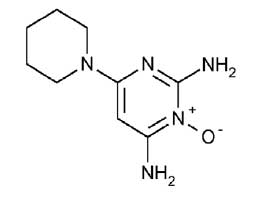
MINOXIDIL TABLETS USP (Minoxidil) is an antihypertensive that acts mainly by causing direct peripheral vasodilatation of the arterioles. Chemically, minoxidil is 2,4-Diamino-6-piperidinopyrimidine 3-oxide. The molecular formula is C9H15N5O and molecular weight is 209.25.
STRUCTURAL FORMULA :
Its structural formula is :
MINOXIDIL TABLETS USP 2.5 mg and 5 mg are white coloured, circular, biconvex tablets having score line on one side and other side “SGP” embossed
COMPOSITION :
Each uncoated tablet contains :
Minoxidil USP 2.5 mg
Excipients q.s.
Each uncoated tablet contains :
Minoxidil USP 5 mg
Excipients q.s.
ACTIONS :
General Pharmacologic Properties :
Minoxidil is an orally effective direct acting peripheral vasodilator that reduces elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance. Microcirculatory blood flow in animals is enhanced or maintained in all systemic vascular beds. In man, forearm and renal vascular resistance decline; forearm blood flow increases while renal blood flow and glomerular filtration rate are preserved. Because it causes peripheral vasodilation, minoxidil elicits a number of predictable reactions. Reduction of peripheral arteriolar resistance and the associated fall in blood pressure trigger sympathetic, vagal inhibitory, and renal homeostatic mechanisms, including an increase in renin secretion, that lead to increased cardiac rate and output and salt and water retention. These adverse effects can usually be minimized by concomitant administration of a diuretic and a beta-adrenergic blocking agent or other sympathetic nervous system suppressant. Minoxidil does not interfere with vasomotor reflexes and therefore does not produce orthostatic hypotension. The drug does not enter the central nervous system in experimental animals in significant amounts, and it does not affect CNS function in man.
Effects on Blood Pressure and Target Organs :
The extent and time-course of blood pressure reduction by minoxidil do not correspond closely to its concentration in plasma. After an effective single oral dose, blood pressure usually starts to decline within one-half hour, reaches a minimum between 2 and 3 hours and recovers at an arithmetically linear rate of about 30 % per day. The total duration of effect is approximately 75 hours. When minoxidil is administered chronically, once or twice a day, the time required to achieve maximum effect on blood pressure with a given daily dose is inversely related to the size of the dose. Thus, maximum effect is achieved on 10 mg/day within 7 days, on 20 mg/day within 5 days and on 40 mg/day within 3 days. The blood pressure response to minoxidil is linearly related to the logarithm of the dose administered. The slope of this log-linear dose-response relationship is proportional to the extent of hypertension and approaches zero at a supine diastolic blood pressure of approximately 85 mm Hg. When used in severely hypertensive patients resistant to other therapy, frequently with an accompanying diuretic and beta-blocker, minoxidil tablets usually decreased the blood pressure and reversed encephalopathy and retinopathy.
PHARMACOKINETICS :
About 90 % of an oral dose of minoxidil has been reported to be absorbed from the gastrointestinal tract. The plasma half-life is about 4.2 hours although the haemodynamic effect may persist for up to 75 hours, presumably due to accumulation at its site of action. Minoxidil is not bound to plasma proteins. It is distributed into breast milk. Minoxidil is extensively metabolised by the liver. It requires sulfation to become active, but the major metabolite is a glucuronide conjugate. Minoxidil is excreted predominantly in the urine mainly in the form of metabolites. Minoxidil and its metabolites are dialysable, although the pharmacological effect is not reversed.
INDICATIONS :
MINOXIDIL TABLETS USP is indicated for the treatment of severe hypertension that is symptomatic or associated with target organ damage. It is indicated for the treatment of hypertension not controlled adequately by a combination of a diuretic and a sympathetic suppressant agent such as a beta blocker. Additionally, it is indicated in hypertension that is not manageable with maximum therapeutic doses of a diuretic plus two other antihypertensive drugs. It should not be used as the sole agent to initiate therapy. It is a peripheral vasodilator and should be given in conjunction with a diuretic, to control salt and water retention, and a beta-adrenergic blocking agent, or appropriate substitute, to control reflex tachycardia.
Administration :
MINOXIDIL TABLETS USP are for oral administration.
Dosage :
Adults and Patients over 12 years of age : An initial daily dose of 5 mg, which may be given as a single or divided dosage, is recommended. The dose can be increased to 20 mg and later 40 mg/day in single or divided dosages, if required for optimum blood pressure control. Dosage adjustments should be made at intervals of not less than three days, until optimum control of blood pressure is achieved. It is seldom necessary to exceed 50 mg per day although, in exceptional circumstances, doses up to 100 mg per day have been used. Twice-daily dosage is satisfactory. Where diastolic pressure reduction of less than 30 mm Hg is required, once daily dosing has been reported as effective.
Children :
For patients of 12 years of age or under, the initial dose should be 200 micrograms per kilogram (0.2 mg/kg) given as a single daily dosage. Incremental increases of 100 - 200 micrograms per kilogram (0.1-0.2 mg/kg) in the daily dose are recommended at intervals of not less than three days until optimum blood pressure control has been achieved, or the maximum daily dose of 1.0 mg/kg has been reached. The maximum recommended dose is 50 mg per day. Experience in children has been limited. The recommendations can be considered only as a rough guide to treatment at present and careful titration is essential.
Rapid reduction of blood pressure :
Under hospital monitoring conditions, rapid reduction of blood pressure can be achieved using continuous blood pressure monitoring and incremental doses of 5 mg every six hours.
Concomitant antihypertensive therapy :
It is recommended that, where possible, antihypertensive therapy, other than a beta-adrenergic blocking agent and a diuretic be discontinued before MINOXIDIL TABLETS USP treatment is started. It is recognised that some antihypertensive agents should not be abruptly discontinued. These drugs should be gradually discontinued during the first week of MINOXIDIL TABLETS USP treatment.
MINOXIDIL TABLETS USP causes sodium retention and if used alone can result in several hundred milli-equivalents of salt being retained together with a corresponding volume of water. Therefore, in all patients who are not on dialysis, MINOXIDIL TABLETS USP must be given in conjunction with a diuretic in sufficient dosage to maintain salt and water balance. Examples of the daily dosages of diuretics commonly used when starting therapy with MINOXIDIL TABLETS USP include :
Hydrochlorothiazide (100 mg) - or other thiazides at equi-effective dosage.
Chlorthalidone (100 mg).
Furosemide (80 mg).
If excessive water retention results in a weight gain of more than 3 pounds when a thiazide or chlorthalidone is being used, diuretic therapy should be changed to furosemide, the dose of which may be increased in accordance with the patient’s requirements. Diuretic dosage in children should be proportionally less in relation to weight. Patients will require a sympathetic nervous system suppressant to limit a MINOXIDIL TABLETS USP induced rise in heart rate. The preferred agent is a beta-blocker equivalent to an adult propranolol dosage of 80 - 160 mg/day. Higher doses may be required when pre-treated patients have an increase in heart rate exceeding 20 beats per minute or when simultaneous introduction causes an increase exceeding 10 beats per minute. When beta-blockers are contra-indicated, alternatives such as methyldopa (250 to 750 mg b.i.d) may be used instead and should be started 24 hours prior to MINOXIDIL TABLETS USP.
Elderly patients :
At present there are no extensive clinical studies with MINOXIDIL TABLETS USP in patients over age 65. There is data indicating that elevated systolic and diastolic pressures are important risk factors for cardiovascular disease in individuals over age 65. However, elderly patients may be sensitive to the blood pressure lowering effect of MINOXIDIL TABLETS USP and thus caution is urged in initiating therapy as orthostatic hypotension may occur. It is suggested that 2.5 mg per day be used as the initial starting dose in patients over 65 years of age.
CONTRAINDICATIONS :
MINOXIDIL TABLETS USP are contraindicated in pheochromocytoma, because it may stimulate secretion of catecholamines from the tumor through its antihypertensive action.
MINOXIDIL TABLETS USP is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
MINOXIDIL TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
Salt and Water Retention :
Congestive Heart Failure - concomitant use of an adequate diuretic is required - MINOXIDIL TABLETS USP must usually be administered concomitantly with a diuretic adequate to prevent fluid retention and possible congestive heart failure; a high ceiling (loop) diuretic is almost always required. Body weight should be monitored closely. If minoxidil is used without a diuretic, retention of several hundred milli-equivalents of salt and corresponding volumes of water can occur within a few days, leading to increased plasma and interstitial fluid volume and local or generalized oedema. Diuretic treatment alone, or in combination with restricted salt intake, will usually minimize fluid retention, although reversible oedema did develop in approximately 10 % of non-dialysis patients so treated. Ascites has also been reported. Diuretic effectiveness was limited mostly by disease-related impaired renal function. The condition of patients with pre-existing congestive heart failure occasionally deteriorated in association with fluid retention although because of the fall in blood pressure (reduction of afterload), more than twice as many improved than worsened. Rarely refractory fluid retention may require discontinuation of minoxidil. Provided that the patient is under close medical supervision, it may be possible to resolve refractory salt retention by discontinuing minoxidil for 1 to 2 days and then resuming treatment in conjunction with vigorous diuretic therapy.
Concomitant Treatment to Prevent Tachycardia is Usually Required :
Minoxidil increases the heart rate. Angina may worsen or appear for the first time during minoxidil treatment, probably because of the increased oxygen demands associated with increased heart rate and cardiac output. The increase in rate and the occurrence of angina generally can be prevented by the concomitant administration of a beta-adrenergic blocking drug or other sympathetic nervous system suppressant. The ability of beta-adrenergic blocking agents to minimize papillary muscle lesions in animals is further reason to utilize such an agent concomitantly. Round-the-clock effectiveness of the sympathetic suppressant should be ensured.
Pericarditis, Pericardial Effusion and Tamponade :
There have been reports of pericarditis occurring in association with the use of minoxidil. The relationship of this association to renal status is uncertain. Pericardial effusion, occasionally with tamponade, has been observed in about 3 % of treated patients not on dialysis, especially those with inadequate or compromised renal function. Although in many cases, the pericardial effusion was associated with a connective tissue disease, the uraemic syndrome, congestive heart failure, or marked fluid retention, there have been instances in which these potential causes of effusion were not present. Patients should be observed closely for any suggestion of a pericardial disorder, and echocardiographic studies should be carried out if suspicion arises. More vigorous diuretic therapy, dialysis, pericardiocentesis, or surgery may be required. If the effusion persists, withdrawal of minoxidil should be considered in light of other means of controlling the hypertension and the patient’s clinical status.
Interaction with Guanethidine :
Although minoxidil does not itself cause orthostatic hypotension, its administration to patients already receiving guanethidine can result in profound orthostatic effects. If at all possible, guanethidine should be discontinued well before minoxidil is begun. Where this is not possible, minoxidil therapy should be started in the hospital and the patient should remain institutionalized until severe orthostatic effects are no longer present or the patient has learned to avoid activities that provoke them.
PRECAUTIONS :
General : Monitor fluid and electrolyte balance and body weight : (see WARNINGS : Salt and Water Retention).
Observe patients for signs and symptoms of pericardial effusion : (see WARNINGS : Pericarditis, Pericardial Effusion and Tamponade). Use after myocardial infarction : MINOXIDIL TABLETS USP have not been used in patients who have had a myocardial infarction within the preceding month. It is possible that a reduction of arterial pressure with minoxidil might further limit blood flow to the myocardium, although this might be compensated by decreased oxygen demand because of lower blood pressure.
Hypersensitivity : Possible hypersensitivity to minoxidil, manifested as a skin rash, has been seen in less than 1 % of patients ; whether the drug should be discontinued when this occurs depends on treatment alternatives.
Renal failure or dialysis : patients may require smaller doses of minoxidil and should have close medical supervision to prevent exacerbation of renal failure or precipitation of cardiac failure. MINOXIDIL TABLETS USP should be used cautiously in diabetic patients.
Pregnancy : Category C
There is limited data from the use of MINOXIDIL TABLETS USP in pregnant women. Studies in animals have shown reproductive toxicity (In a fertility study with male and female rats, a dose-dependent reduction of the conception rate was found. The doses corresponded to one to five times the maximum dose used in humans to treat hypertension. Teratogenicity has been demonstrated in the rat at doses above 80 mg/kg/day. Teratogenicity was not demonstrated in the rabbit). MINOXIDIL TABLETS USP is not recommended during pregnancy and in women of child bearing potential not using contraception.
Lactation :
There is insufficient information on the excretion of minoxidil in human milk. A risk to the suckling child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from minoxidil therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.

 Cardiovascular
Cardiovascular