
1 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
HYDROXOCOBALAMIN INJECTION B.P. (Hydroxocobalamin Acetate) is Vitamin B12 analogue. Chemically, Hydroxocobalamin Acetate is Coa-[a-(5,6-dimethylbenzimidazolyl)]-Cob-hydroxocobamide acetate. The molecular formula is C64H93CoN13O17P and molecular weight is 1406.
STRUCTURAL FORMULA :
Its structural formula is :
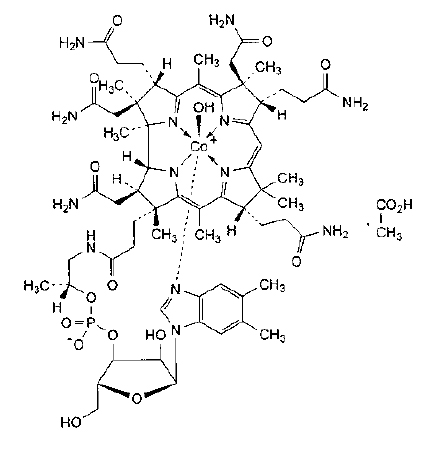
HYDROXOCOBALAMIN INJECTION B.P. is a sterile, clear red colour solution filled in ampoule of suitable size.
COMPOSITION :
Each ml contains :
Hydroxocobalamin Acetate B.P.
equivalent to anhydrous Hydroxocobalamin 1.0 mg
Sodium Chloride B.P. 8.20 mg
Methyl Hydroxybenzoate B.P. 0.15 % w/v
Propyl Hydroxybenzoate B.P. 0.02 % w/v
(as preservatives)
Water for Injections B.P. q.s.
Adequate overages of vitamin added to compensate for the loss on storage.
ACTIONS :
Vitamin B12 is essential to growth, cell reproduction, haematopoiesis, nucleoprotein and myelin synthesis.
PHARMACOKINETICS :
Fifty percent of the administered dose of hydroxocobalamin disappears from the injection site in 2.5 hours. Hydroxocobalamin is bound to plasma proteins and stored in the liver. It is excreted in the bile and undergoes some enterohepatic recycling. Within 72 hours after injection of 500 to 1000 mcg of hydroxocobalamin, 16 to 66 percent of the injected dose may appear in the urine. The major portion is excreted within the first 24 hours. An intramuscular injection of hydroxocobalamin produced higher serum levels than the same dose of cyanocobalamin and these levels are well maintained.
INDICATIONS :
HYDROXOCOBALAMIN INJECTION B.P. is indicated for the treatment of :
- Pernicious anaemia and other macrocytic anaemias without neurological involvement.
- Prophylaxis of macrocytic anaemias associated with
- vitamin B12 deficiency.
- Tobacco amblyopia and Leber’s optic atrophy.
- Congenital transcobalamin II deficiency.
- Methylmalonic acidaemia and homocystinuria.
Administration :
For Intramuscular Use Only.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults :
Pernicious anaemia and other macrocytic anaemias without neurological involvement :
Initially 1 mg 3 times a week for 2 weeks then 1 mg every 3 months.
Pernicious anaemia and other macrocytic anaemias with neurological involvement :
Initially 1 mg on alternate days until no further improvement, then 1 mg every 2 months.
Prophylaxis of macrocytic anaemias associated with vitamin B12 deficiency :
1 mg every 2 - 3 months.
Tobacco amblyopia and Leber’s optic atrophy :
Initially 1 mg daily for 2 weeks, then 1 mg twice weekly until no further improvement, thereafter 1 mg every 1 - 3 months.
Paediatric population :
Macrocytic anaemia without neurological involvement :
Child 1 month - 18 years :
Initially 250 micrograms - 1 mg 3 times a week for 2 weeks then 250 micrograms once weekly until blood count normal, then 1 mg every 3 months.
Macrocytic anaemia with neurological involvement :
Child 1 month - 18 years :
Initially 1 mg on alternate days until no further improvement, then 1 mg every 2 months.
Prophylaxis of macrocytic anaemias associated with vitamin B12 deficiency :
Child 1 month - 18 years :
1 mg every 2 - 3 months.
Leber’s optic atrophy :
Initially 1 mg daily for 2 weeks, then 1 mg twice weekly until no further improvement, thereafter 1 mg every 1 - 3 months.
Congenital transcobalamin II deficiency :
Neonate :
1 mg 3 times a week, reduce after 1 year to 1 mg once weekly or as appropriate.
Child 1 month - 18 years :
1 mg 3 times a week, reduce after 1 year to 1 mg once weekly or as appropriate.
Methylmalonic acidaemia and homocystinuria :
Child 1 month - 18 years :
Initially 1 mg daily for 5 - 7 days, reduce according to response to maintenance dose of up to 1 mg once or twice weekly.
CONTRAINDICATIONS :
Hypersensitivity to any ingredient in the preparation.
Avoid the intravenous route. Folic acid is not a substitute for vitamin B12 although it may improve vitamin B12 deficient megaloblastic anaemia. Exclusive use of folic acid in treating vitamin B12 deficient megaloblastic anaemia could result in progressive and irreversible neurologic damage. Blunted or impeded therapeutic response to vitamin B12 may be due to such conditions as infection, uraemia, drugs having bone marrow suppressant properties such as chloramphenicol, and concurrent iron or folic acid deficiency.
PRECAUTIONS :
General :
The validity of diagnostic vitamin B12 or folic acid blood assays could be compromised by medications, and this should be considered before relying on such tests for therapy. Vitamin B12 is not a substitute for folic acid and since it might improve folic acid deficient megaloblastic anaemia, indiscriminate use of vitamin B12 could mask the true diagnosis. Hypokalaemia and thrombocytosis could occur upon conversion of severe megaloblastic to normal erythropoiesis with B12 therapy. Therefore, serum potassium levels and the platelet count should be monitored carefully during therapy. Vitamin B12 deficiency may suppress the signs of polycythaemia vera. Treatment with vitamin B12 may unmask this condition. The dosage schemes given above are normally satisfactory, but regular examination of the blood is advisable. If megaloblastic anaemia fails to respond, folate metabolism should be investigated. Doses in excess of 10 micrograms daily may produce a haematological response in patients with folate deficiency. Indiscriminate administration may mask the true diagnosis. Cardiac arrhythmias secondary to hypokalaemia during initial therapy have been reported. Plasma potassium should therefore be monitored during this period.
Carcinogenesis, Mutagenesis, Impairment of Fertility :
Studies of carcinogenicity, mutagenesis, or impairment of fertility have not been performed with hydroxocobalamin.
PREGNANCY AND LACTATION :
Pregnancy : Category C
Teratogenic Effects :
Animal reproduction studies have not been conducted with hydroxocobalamin. It is also not known whether hydroxocobalamin can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. Hydroxocobalamin should be given to a pregnant woman only if clearly needed.
Nursing mothers :
Hydroxocobalamin is distributed into breast milk; however, problems in humans have not been documented with intake of normal daily recommended amounts.
Paediatric Use :
Problems in paediatrics have not been documented with intake of normal daily recommended amounts.
INTERACTIONS AND INCOMPATIBILITIES :
Chloramphenicol-treated patients may respond poorly to Hydroxocobalamin injection. Serum concentrations of hydroxocobalamin may be lowered by oral contraceptives. The above interactions are unlikely to have clinical significance. Antimetabolites and most antibiotics invalidate Vitamin B12 assays by microbiological techniques.
SIDE EFFECTS :
Nausea, headache, dizziness; fever, hypersensitivity reactions (including rash and pruritus); injection-site reactions; hypokalaemia and thrombocytosis during initial treatment; chromaturia.
INFORMATION FOR PATIENTS :
For adults, the daily requirement of vitamin B12 is probably about 1 to 2 micrograms and this amount is present in most normal diets. Vitamin B12 occurs only in animal products; it does not occur in vegetables, therefore strict vegetarian (vegan) diets that exclude dairy products may provide an inadequate amount although it has been said that many years of vegetarianism are necessary before a deficiency is produced, if at all. Meats, especially liver and kidney, milk, eggs, and other dairy products, and fish are good sources of vitamin B12.
OVERDOSAGE :
The intravenous LD50 of hydroxocobalamin in mice is greater than 50 ml/kg.
TREATMENT OF OVERDOSAGE :
Treatment is unlikely to be required in the case of overdosage.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
HYDROXOCOBALAMIN INJECTION B.P. contains Hydroxocobalamin Acetate B.P. equivalent to anhydrous Hydroxocobalamin 1 mg in 1 ml aqueous solution.
10 Ampoules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





