50 mg, 150 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
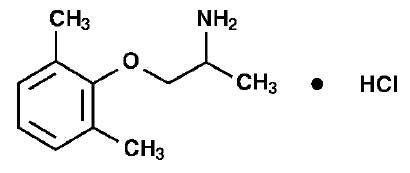
LITIMEX (Mexiletine Hydrochloride) is a class 1B anti-arrhythmic agent based on the Vaughan-Williams classification 1 with local anaesthetic properties, similar in structure and activity to lignocaine. Chemically, Mexiletine Hydrochloride is 2-Propanamine, 1-(2,6-dimethylphenoxy)-, hydrochloride, (±)-. The molecular formula is C11H17NO·HCl and molecular weight is 215.72.
STRUCTURAL FORMULA :
Its structural formula is :
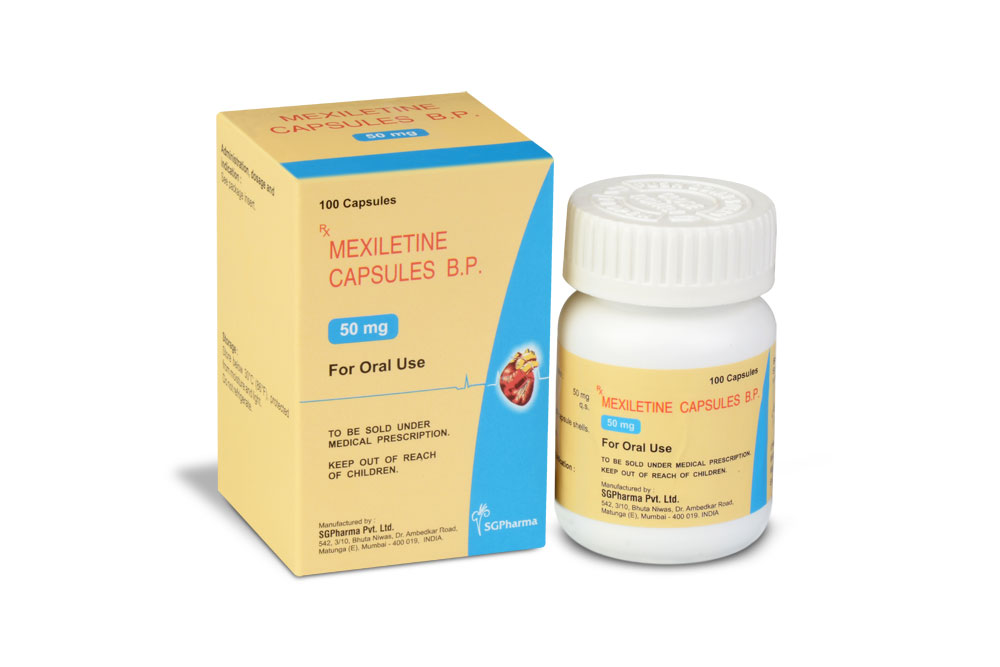
LITIMEX 50 is a hard gelatin capsule of red/purple colour.
LITIMEX 150 is a hard gelatin capsule of red/red colour.
COMPOSITION :
LITIMEX 50
Each hard gelatin capsule contains :
Mexiletine Hydrochloride USP 50 mg
Excipients q.s.
Approved colours used in empty capsule shells
LITIMEX 150
Each hard gelatin capsule contains :
Mexiletine Hydrochloride USP 150 mg
Excipients q.s.
Approved colours used in empty capsule shells
ACTIONS :
LITIMEX (Mexiletine Hydrochloride) is a Class 1B anti-arrhythmic agent based on the Vaughan Williams classification with local anaesthetic properties, similar in structure and activity to lidocaine (lignocaine). Mexiletine depresses the maximum rate of depolarisation with little or no modification of resting potentials or the duration of action potentials.
Administration :
Capsules should be swallowed whole with ample liquid, preferably with the patient in an upright position. It is advisable to take LITIMEX after food.
Dosage :
Plasma elimination half-life may be prolonged in moderate to severe hepatic disease, and in patients with a creatinine clearance of less than 10 ml/min; individual dose titration is advised in these conditions. The dosage of LITIMEX must be individualised on the basis of response and tolerance, both of which are dose related.
1. Loading dose : Initially, if more rapidly effective blood levels are required, a loading dose, usually 400 mg may be desirable.
2. Maintenance dose : Give 200-250 mg LITIMEX three to four times daily commencing 2 hours after the loading dose. The usual daily dose is between 600-800 mg in divided doses; optimal doses range from 300-1200 mg daily in divided doses.
Alternative loading dose regimes :
1. Combination IV Litimex and oral Litimex loading dose : IV injection of 8 ml (200 mg) LITIMEX given at a suggested rate of 1 ml per minute. On completion of injection or infusion give 400 mg Litimex orally.
Maintenance dose : As in Maintenance dose.
2. Combination IV lidocaine (lignocaine) and oral Litimex loading dose : Give IV lidocaine (lignocaine) according to manufacturers instructions. On completion of injection give 400 mg Litimex orally.
Maintenance dose : As in Maintenance dose.
3. Change over from IV to oral maintenance : On discontinuing the IV infusion commence the maintenance dose. The first capsule should be taken at, or shortly before, the end of the infusion (an oral loading dose should not be given). Give 200 - 250 mg LITIMEX orally three or four times a day.
NOTES :
1.The loading dose regime is designed to compensate for the rapid phase of tissue distribution which occurs especially with IV loading.
2. If the optimum therapeutic effect is not achieved, the rate of infusion or oral dosage may be increased, side effects permitting.
3.Gastric emptying time may be delayed in patients with myocardial infarction and/or to whom opiates have been given and thus, it may be necessary to titrate the dose against therapeutic effects and side effects.
4. The 50 mg capsule is available in order that a more precise dose titration may be undertaken should this be required. Small increments will also reduce the incidence of side effects.
5.When mexiletine therapy is commenced, patients should be monitored closely (ECG, blood pressure and routine laboratory tests) over a period of at least 24 hours, and dosage adjustment made on the basis of this. Monitoring is particularly recommended in the following situations : sinus node dysfunction, conduction defects, bradycardia, hypotension or cardiac, renal or hepatic failure. There may be a potentiation of tremor in patients with Parkinsonism.
Regular monitoring of cardiac function throughout treatment is advisable. The duration of treatment required in any patient is of necessity variable, and although no precise guide can be given, withdrawal of treatment may be attempted after a suitable period free of arrhythmia. Gradual withdrawal i.e. over 1-2 weeks, is preferable as arrhythmias which have been satisfactorily controlled may recur. No specific information on the use of this product in the elderly or children is available. Clinical trials have included patients over 65 years and no adverse reactions specific to this age group have been reported.
CONTRAINDICATIONS :
LITIMEX should not be used in the first three months following myocardial infarction or where cardiac output is limited (left ventricular ejection fraction of less than 35 %), except in patients with life-threatening ventricular arrhythmias.
LITIMEX is contraindicated in the presence of cardiogenic shock or pre-existing second or third degree A-V block if no pacemaker is present. LITIMEX should not be used in patients known to be hypersensitive to the active ingredient or one of the excipients of the product, or to local anaesthetics (e.g. lignocaine).
WARNINGS AND PRECAUTIONS :
If LITIMEX is used in the following situations the patient should be closely monitored and dosage reduced if necessary : sinus node dysfunction, conduction defect, bradycardia, hypotension or cardiac failure. Patients with uncompensated liver cirrhosis show evidence of delayed breakdown and elimination rates of LITIMEX. This may also occur in patients with severe renal failure. In these patients, the dose must be adjusted on an individual basis. Patients in whom pathologically high liver values have been established or who have signs or symptoms of impaired liver function, should be monitored carefully. Careful monitoring is also recommended in patients with convulsive disorders. Even if LITIMEX is used as prescribed, it can influence reactions to such an extent that the ability to drive or operate machinery is limited. This applies to an even greater extent in combination with alcohol.
Pregnancy :
Mexiletine freely crosses the placenta. Therefore, LITIMEX should only be used in pregnancy if the potential benefit justifies the potential risk.
Lactation :
LITIMEX appears in breast milk in concentrations, which may have an effect on the infant. Therefore, if the use of LITIMEX is deemed essential for the mother, an alternative method of infant feeding should be considered.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
LITIMEX may impair the ability to drive or operate machinery, especially when taken in combination with alcohol.
INTERACTIONS :
Where there is a concurrent administration of LITIMEX and other antiarrhythmic drugs, an increased effect on conduction and contractility of the heart is to be expected. Combinations with propranolol, quinidine and amiodarone have been used. All medicines which affect gastrointestinal movement may affect the absorption of oral LITIMEX. Opiates may delay the entry of LITIMEX into the bloodstream. Since LITIMEX is metabolised mainly in the liver, substances that influence liver enzyme function may alter the concentration of LITIMEX in the blood. In particular, interactions with the two cytochrome P450 isoenzymes CYP1A2 and CYP2D6 have to be considered. It may be necessary to reduce the dose of LITIMEX in cases of concomitant administration of substances that lead to enzyme inhibition in the liver. In cases of concurrent therapy with substances that lead to enzyme induction it may be necessary to increase the dose of LITIMEX since it is metabolised at a faster rate. In cases of concurrent therapy with LITIMEX the serum level of theophylline rises. The same applies to caffeine. Drugs which markedly acidify or alkalise urine should be avoided because they may enhance or reduce (respectively) the rate of drug excretion and the plasma concentration of mexiletine. An increased tendency to bleed has been observed when LITIMEX has been administered to some patients stabilised on Warfarin. Local anaesthetic toxicity may occur in patients who receive LITIMEX and local anaesthetic agents concurrently.
SIDE EFFECTS :
Side-effects are mainly related to blood concentration and may therefore be seen during the initial phases of both IV and oral treatment when fluctuation may occur before the blood and tissue concentrations reach equilibrium. Reducing the rate of injection of infusion or delaying the next oral dose allows the blood concentration to fall and usually reduces side-effects.
Generally side-effects are of five types :
Gastro-Intestinal : Nausea, vomiting, indigestion, constipation, diarrhoea, dry mouth, unpleasant taste, hiccoughs. Oesophageal ulceration may occur if oral LITIMEX is swallowed without adequate liquid and is lodged in the oesophagus.
Central Nervous System :
Drowsiness, dizziness, diplopia, blurred vision, nystagmus, dysarthria, ataxia, tremor, paraesthesiae, convulsion, psychiatric disorders, confusional state, insomnia.
Cardiovascular :
Hypotension, sinus bradycardia, atrial fibrillation, palpitation and conduction defects. Exacerbation of arrhythmias, pre-existing heart failure and torsade de pointes. When hypotension has occurred this has tended to be in patients with severe illness who have already been given a variety of anti-arrhythmic or other preparations and, if associated with bradycardia, may be reduced by the use of atropine. Pulmonary infiltrates, interstitial lung disease and pulmonary fibrosis have been observed in isolated cases.
Haematological :
Rash, arthralgia, fever, thrombocytopenia and appearance of positive but symptomless antinuclear factor titres. Leucopenia has been observed rarely. Rare cases of Stevens-Johnson syndrome, some with liver involvement, have been reported in Japan. Isolated cases of erythroderma have also been reported.
Hepatic :
Liver damage has been observed following Mexiletine HCl Capsules administration; jaundice has been reported.
OVERDOSAGE :
The minimum fatal dose is unknown but 4.40 g proved fatal in a healthy young adult.
The clinical features include : Nausea, vomiting, drowsiness, confusion, ataxia and convulsions. Blurred vision and paraesthesiae have also been reported. Hypotension, sinus bradycardia, atrial fibrillation and cardiac arrest are more specific effects.
TREATMENT OF OVERDOSAGE :
General symptomatic treatment is advisable. Gastric lavage should be performed where appropriate and the patient should be transferred to an intensive / coronary care unit for possible cardio-pulmonary support. Arrhythmias should be treated as appropriate and intravenous diazepam may be useful to control convulsions. In the event of serious bradycardia and hypotension it is advisable to first administer an IV dose of 0.5 - 1.0 mg atropine.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
36 months from the date of manufacture.
PRESENTATION :
LITIMEX 50 / LITIMEX 150 contains Mexiletine Hydrochloride USP 50 mg & 150 mg respectively.
10 Blisters of 10 capsules per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular