
5 mg, 10 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
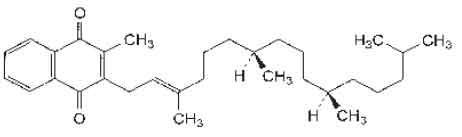
PHYTOMENADIONE TABLETS B.P. (Phytomenadione – Vitamin K1) is a vitamin occurring naturally in plants (as phylloquinone) as well as produced synthetically. Chemically, Phytomenadione is 2-Methyl-3-phytyl-1, 4-naphthoquinone. The molecular formula is C31H46O2 and molecular weight is 450.7.
STRUCTURAL FORMULA :
Its structural formula is :
PHYTOMENADIONE TABLETS B.P. is yellow coloured, circular, biconvex tablet with breakline on one side and plain on other side.
COMPOSITION :
Each uncoated tablet contains :
Diluted Phytomenadione SD Powder
equivalent to Phytomenadione B.P. 5 mg
Excipients q.s.
Colour : Tartrazine
Contains added synthetic flavour.
Each uncoated tablet contains :
Diluted Phytomenadione SD Powder
equivalent to Phytomenadione B.P. 10 mg
Excipients q.s.
Colour : Tartrazine
Contains added synthetic flavour.
ACTIONS :
PHYTOMENADIONE TABLETS B.P. possess the same type and degree of activity as does naturally-occurring vitamin K, which is necessary for the production via the liver of active prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). The prothrombin test is sensitive to the levels of three of these four factors – II, VII, and X. Vitamin K is an essential cofactor for a microsomal enzyme that catalyzes the posttranslational carboxylation of multiple, specific, peptide-bound glutamic acid residues in inactive hepatic precursors of factors II, VII, IX, and X. The resulting gamma-carboxyglutamic acid residues convert the precursors into active coagulation factors that are subsequently secreted by liver cells into the blood. In normal animals and humans, phytomenadione is virtually devoid of pharmacodynamic activity. However, in animals and humans deficient in vitamin K, the pharmacological action of vitamin K is related to its normal physiological function; that is, to promote the hepatic biosynthesis of vitamin K-dependent clotting factors.
PHARAMACOKINETICS :
Absorption :
Oral doses of phytomenadione are absorbed primarily from the middle portions of the small intestine. Optimal absorption requires the presence of bile and pancreatic juice. Systemic availability following oral or intramuscular dosing is approximately 50 %, with a wide range of interindividual variability. Onset of action occurs approximately 1-3 hours after intravenous administration and 4-6 hours after intramuscular or oral doses.
Distribution :
The primary distribution compartment corresponds to the plasma volume. In blood plasma 90 % of phytomenadione is bound to lipoproteins (VLDL fraction). Normal plasma concentrations of phytomenadione range from 0.4 to 1.2 μg per litre. Plasma concentrations between 10 and 20 μg per litre are achieved following intramuscular doses of 10 g phytomenadione. Phyotomenadione does not readily cross the placenta and is poorly distributed into breast milk.
Metabolism :
Phytomenadione is rapidly converted into more polar metabolites, including phytomenadione-2,3 epoxide. Some of this metabolite is reconverted into phytomenadione.
Elimination :
Following metabolic degradation, phytomenadione is excreted in the bile and urine as glucuronide and sulfate conjugates. Less than 10 % of a dose is excreted unchanged in the urine. The elimination half-life has been reported to be between 1.5 and 3 hours.
Pharmacokinetics in Special Clinical Situations :
Absorption of phytomenadione is impaired by various conditions, including malabsorption syndromes, short bowel syndrome, biliary atresia and pancreatic insufficiency. Elderly anticoagulated patients are more sensitive than younger ones to parenteral phytomenadione.
INDICATIONS :
PHYTOMENADIONE TABLETS B.P. is indicated in the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity.
PHYTOMENADIONE TABLETS B.P. are indicated in :
- Anticoagulant-induced prothrombin deficiency caused by coumarin or indanedione derivatives;
- Hypoprothrombinaemia secondary to antibacterial therapy;
- Hypoprothrombinaemia secondary to administration of salicylates;
- Hypoprothrombinaemia secondary to obstructive jaundice or biliary fistulas but only if bile salts are administered concurrently, since otherwise the oral vitamin K will not be absorbed.
Administration :
PHYTOMENADIONE TABLETS B.P. are for oral administration and should be chewed or allowed to dissolve slowly in the mouth.
Anticoagulant - Induced Prothrombin Deficiency in Adults :
To correct excessively prolonged prothrombin times caused by oral anticoagulant therapy – 2.5 to 10 mg or up to 25 mg initially is recommended. In rare instances 50 mg may be required, Frequency and amount of subsequent doses should be determined by prothrombin time response or clinical condition. If, in 12 to 48 hours after oral administration, the prothrombin time has not been shortened satisfactorily, the dose should be repeated.
Hypoprothrombinaemia Due to Other Causes in Adults :
If possible, discontinuation or reduction of the dosage of drugs interfering with coagulation mechanisms (such as salicylates, antibiotics) is suggested as an alternative to administering concurrent PHYTOMENADIONE TABLETS B.P. The severity of the coagulation disorder should determine whether the immediate administration of PHYTOMENADIONE TABLETS B.P. is required in addition to discontinuation or reduction of interfering drugs. A dosage of 2.5 to 25 mg or more (rarely up to 50 mg) is recommended, the amount and route of administration depending upon the severity of the condition and response obtained. The oral route should be avoided when the clinical disorder would prevent proper absorption. Bile salts must be given with the tablets when the endogenous supply of bile to the gastrointestinal tract is deficient.
CONTRAINDICATIONS :
Hypersensitivity to any component of this medication. PHYTOMENADIONE TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
An immediate coagulant effect should not be expected after administration of phytomenadione. Phytomenadione will not counteract the anticoagulant action of heparin. When vitamin K1 is used to correct excessive anticoagulant-induced hypoprothrombinaemia, anticoagulant therapy still being indicated, the patient is again faced with the clotting hazards existing prior to starting the anticoagulant therapy. Phytomenadione is not a clotting agent, but overzealous therapy with vitamin K1 may restore conditions which originally permitted thromboembolic phenomena. Dosage should be kept as low as possible, and prothrombin time should be checked regularly as clinical conditions indicate.
Repeated large doses of vitamin K are not warranted in liver disease if the response to initial use of the vitamin is unsatisfactory. Failure to respond to vitamin K may indicate a congenital coagulation defect or that the condition being treated is inherently unresponsive to vitamin K. The tablet contains “tartrazine” colour which may cause allergic reactions, including bronchial asthma, especially in patients who are allergic to the acetyl salicylic acid.
PRECAUTIONS :
General :
Vitamin K1 is fairly rapidly degraded by light; therefore, always protect PHYTOMENADIONE TABLETS B.P. from light. Store PHYTOMENADIONE TABLETS B.P. in closed original carton until contents have been used.
Laboratory Tests :
Prothrombin time should be checked regularly as clinical conditions indicate. PHYTOMENADIONE TABLETS B.P. should be used cautiously in diabetic patients.
Pregnancy : Category C
Animal reproduction studies have not been conducted with PHYTOMENADIONE TABLETS B.P. It is also not known whether PHYTOMENADIONE TABLETS B.P. can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. PHYTOMENADIONE TABLETS B.P. should be given to pregnant woman only if clearly needed.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PHYTOMENADIONE TABLETS B.P. is administered to a nursing woman.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established with PHYTOMENADIONE TABLETS B.P. Haemolysis, jaundice, and hyperbilirubinaemia in newborns, particularly in premature infants, have been reported with vitamin K.
INTERACTIONS AND INCOMPATIBILITIES :
Temporary resistance to prothrombin-depressing anticoagulants may result, especially when larger doses of phytomenadione are used. If relatively large doses have been employed, it may be necessary when reinstituting anticoagulant therapy to use somewhat larger doses of the prothrombin-depressing anticoagulant or to use one which acts on a different principle, such as heparin sodium.
SIDE EFFECTS :
Cardiovascular :
Hypotension; cyanosis.
Central Nervous System :
Headache; dizziness.
Dermatologic :
Pruritic erythematous plaques at IM injection site; rash; urticaria.
Hepatic :
Hyperbilirubinemia, including kernicterus, in newborns.
Others :
Anaphylactoid reactions; pain, swelling and tenderness at injection site; death after IV injection.
OVERDOSAGE :
The intravenous and oral LD50s in the mouse are approximately 1.17 g/kg and greater than 24.18 g/kg, respectively. Hypervitaminosis of Vitamin K1 is unknown.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
PHYTOMENADIONE TABLETS B.P. contains Diluted Phytomenadione SD Powder equivalent to Phytomenadione B.P. 5 mg / 10 mg.
2 Strips of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





