
8 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
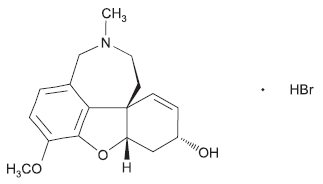
GALANTAMINE TABLETS USP (Galantamine Hydrobromide) is a reversible inhibitor of acetylcholinesterase activity. Chemically, Galantamine Hydrobromide is (4aS,6R,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro [3a,3,2-ef] [2] benzazepin-6-ol hydrobromide. The molecular formula is C17H21NO3 ·HBr and molecular weight is 368.27.
STRUCTURAL FORMULA :
Its structural formula is :
GALANTAMINE TABLETS USP is yellow, circular film coated tablet having plain on both side.
COMPOSITION :
Each film coated tablet contains :
Galantamine Hydrobromide USP
equivalent to Galantamine 8 mg
Excipients q.s.
Colour : Aluminium Lake Quinoline Yellow.
ACTIONS :
Galantamine is a cholinomimetic with a dual mechanism of action. Galantamine is a reversible inhibitor of the enzyme acetylcholinesterase, and enhances the intrinsic action of acetylcholine on nicotinic receptors. The cognitive dysfunction (memory, attention, learning) in dementia of the Alzheimer type is related to the profound dysfunction of the cholinergic neurotransmission system in the brain. Galantamine, a tertiary alkaloid, enhances the efficacy of the physiologically available acetylcholine through a dual mechanism of action : acetylcholinesterase inhibition and nicotinic receptor modulation. Galantamine is a selective, competitive and reversible inhibitor of acetylcholinesterase, the enzyme responsible for the breakdown of the neurotransmitter acetylcholine. As a consequence, the breakdown of acetylcholine, released by the remaining healthy brain cells, is slowed down leaving more neurotransmitter available to support normal brain function. In addition, galantamine enhances the intrinsic action of acetylcholine on nicotinic receptors, probably through binding to an allosteric site of the receptor. Stimulation of nicotinic receptors has been associated with improved cognitive function and neuroprotection against amyloid induced neurotoxicity. Amyloid peptide is the major component of amyloid plaques, one of the hallmarks of Alzheimer’s disease. Modulation of the nicotinic receptor could also lead to enhanced neurotransmitter release, including the release of acetylcholine.
PHARMACOKINETICS :
Galantamine is well absorbed from the gastrointestinal tract. Peak plasma concentrations are reached in about 1 hour after ingestion from conventional formulations; with modified-release formulations; peak concentrations occur about 4 to 5 hours after a dose and are somewhat lower. The absolute oral bioavailability of galantamine is about 90 %. The presence of food delays the rate of absorption although the extent of absorption is not affected. Protein binding is about 18 %. Galantamine is partially metabolised by the cytochrome P450 isoenzymes CYP2D6 and CYP3A4; a number of active metabolites are formed. The elimination half-life is about 7 to 8 hours. After 7 days, the majority of a single oral dose is recovered in the urine with up to about 6 % detected in the faeces; about 20 to 30 % of the dose is excreted in the urine as unchanged galantamine. Clearance is reported to be 20 % lower in females than in males and 25 % lower in poor metabolisers than in extensive metabolisers.
INDICATIONS :
GALANTAMINE TABLETS USP is indicated for symptomatic treatment of mild to moderately severe dementia of the Alzheimer type.
Administration :
GALANTAMINE TABLETS USP is for oral administration. GALANTAMINE TABLETS USP should be given twice a day, preferably with morning and evening meals. Ensure adequate fluid intake during treatment.
Dosage :
Adults/ Elderly :
Starting dose :
The recommended starting dose is 8 mg/day (4 mg twice a day) for 4 weeks.
Maintenance dose :
The tolerance and dosing of galantamine should be reassessed on a regular basis, preferably within 3 months after start of treatment. Thereafter, the clinical benefit of galantamine and the patient’s tolerance of treatment should be reassessed on a regular basis. Maintenance treatment can be continued for as long as therapeutic benefit is favourable and the patient tolerates treatment with galantamine. Discontinuation of galantamine should be considered when evidence of a therapeutic effect is no longer present or if the patient does not tolerate treatment. The initial maintenance dose is 16 mg/day (8 mg twice a day) and patients should be maintained on 16 mg/day for at least 4 weeks. An increase to the maintenance dose of 24 mg/day (12 mg twice a day) should be considered on an individual basis after appropriate assessment including evaluation of clinical benefit and tolerability. In individual patients not showing an increased response or not tolerating 24 mg/day, a dose reduction to 16 mg/day should be considered.
Treatment withdrawal :
There is no rebound effect after abrupt discontinuation of treatment (e.g. in preparation for surgery).
Re-initiation of therapy :
If treatment is interrupted for longer than several days, treatment should be re-initiated with the lowest daily dose and gradually increased to the maximum tolerated dose to achieve the desired clinical effect. The incidence and severity of adverse events are generally related to the higher doses of GALANTAMINE TABLETS USP. In patients co-treated with ketoconazole or potent inhibitors of cytochrome P450 2D6, dose reductions can be considered.
Renal impairment :
Galantamine plasma concentrations may be increased in patients with moderate to severe renal impairment. For patients with a creatinine clearance ≥ 9 ml/min, no dosage adjustment is required. In patients with severe renal impairment (creatinine clearance less than 9 ml/min), the use of galantamine is contraindicated.
Hepatic impairment :
Galantamine plasma concentrations may be increased in patients with moderate to severe hepatic impairment. In patients with moderately impaired hepatic function (Child-Pugh score 7-9), based on pharmacokinetic modelling, it is recommended that dosing should begin with 4 mg once daily, preferably taken in the morning, for at least 1 week. Thereafter, patients should proceed with 4 mg twice-daily for at least 4 weeks. In these patients, daily doses should not exceed 8 mg twice daily. In patients with severe hepatic impairment (Child-Pugh score greater than 9), the use of galantamine is contraindicated. No dosage adjustment is required for patients with mild hepatic impairment.
Concomitant treatment :
In patients treated with potent CYP2D6 or CYP3A4 inhibitors dose reductions can be considered.
Paediatric population :
There is no relevant use of galantamine in the paediatric population.
CONTRAINDICATIONS :
GALANTAMINE TABLETS USP is hypersensitivity to the active substance or to any of the excipients. Because no data are available on the use of galantamine in patients with severe hepatic (Child-Pugh score greater than 9) and severe renal (creatinine clearance less than 9 ml/min) impairment, galantamine is contraindicated in these populations. GALANTAMINE TABLETS USP is contra-indicated in patients who have both significant renal and hepatic dysfunction. GALANTAMINE TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
Anaesthesia :
Galantamine, as a cholinesterase inhibitor, is likely to exaggerate the neuromuscular blocking effects of succinylcholine-type and similar neuromuscular blocking agents during anaesthesia.
Cardiovascular Conditions :
Because of their pharmacological action, cholinesterase inhibitors have vagotonic effects on the sinoatrial and atrioventricular nodes, leading to bradycardia and AV block. These actions may be particularly important to patients with supraventricular cardiac conduction disorders or to patients taking other drugs concomitantly that significantly slow heart rate. Postmarketing surveillance of marketed anticholinesterase inhibitors has shown, however, that bradycardia and all types of heart block have been reported in patients both with and without known underlying cardiac conduction abnormalities. Therefore all patients should be considered at risk for adverse effects on cardiac conduction. In randomized controlled trials, bradycardia was reported more frequently in galantamine-treated patients than in placebo-treated patients, but was rarely severe and rarely led to treatment discontinuation. The overall frequency of this event was 2 to 3 % for galantamine doses up to 24 mg/day compared with <1 % for placebo. No increased incidence of heart block was observed at the recommended doses. Patients treated with galantamine up to 24 mg/day using the recommended dosing schedule showed a dose-related increase in risk of syncope (placebo 0.7 % [2/286]; 4 mg BID 0.4 % [3/692]; 8 mg BID 1.3 % [7/552]; 12 mg BID 2.2 % [6/273]).
Gastrointestinal Conditions :
Through their primary action, cholinomimetics may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those with an increased risk for developing ulcers, e.g., those with a history of ulcer disease or patients using concurrent nonsteroidal anti-inflammatory drugs (NSAIDS). Clinical studies of galantamine have shown no increase, relative to placebo, in the incidence of either peptic ulcer disease or gastrointestinal bleeding. Galantamine, as a predictable consequence of its pharmacological properties, has been shown to produce nausea, vomiting, diarrhoea, anorexia, and weight loss.
Genitourinary :
Although this was not observed in clinical trials with galantamine, cholinomimetics may cause bladder outflow obstruction.
Neurological Conditions :
Seizures :
Cholinesterase inhibitors are believed to have some potential to cause generalized convulsions. However, seizure activity may also be a manifestation of Alzheimer’s disease. In clinical trials, there was no increase in the incidence of convulsions with galantamine, compared to placebo.
Use in children :
Use of GALANTAMINE TABLETS USP in children is not recommended. No data on the use of GALANTAMINE TABLETS USP in paediatric patients are available.
Use in other types of dementia :
The benefit of GALANTAMINE TABLETS USP in patients with other types of dementia or other types of memory impairment has not been demonstrated. GALANTAMINE TABLETS USP is indicated for patients with mild to moderately severe dementia of the Alzheimer type.
Serious skin reactions :
Serious skin reactions (Stevens-Johnson syndrome and acute generalized exanthematous pustulosis) have been reported in patients receiving GALANTAMINE TABLETS USP. It is recommended that patients be informed about the signs of serious skin reactions, and that use of GALANTAMINE TABLETS USP be discontinued at the first appearance of skin rash.
Weight monitoring :
Patients with Alzheimer’s disease lose weight. Treatment with cholinesterase inhibitors, including galantamine, has been associated with weight loss in these patients. During therapy, patient’s weight should be monitored. GALANTAMINE TABLETS USP should be used
cautiously diabetic patients.
Pregnancy : Category B1
No studies are available on the use of GALANTAMINE TABLETS USP in pregnant women. GALANTAMINE TABLETS USP should be used with caution during pregnancy and only if the potential benefit justifies the potential risk to the foetus. Reproduction studies in pregnant rats and rabbits at respective exposure levels up to 5 and 3 times higher than that in humans at the maximum recommended dose (based on plasma AUC values), did not show any evidence of teratogenic potential. Minor skeletal abnormalities and decreased birth weight were seen at the highest dose in rats.
Nursing Mothers :
It is not known whether Galantamine is excreted in human breast milk and no studies have been performed in lactating women. Therefore, women taking GALANTAMINE TABLETS USP should not breast-feed.
Paediatric Use :
There are no adequate and well-controlled trials documenting the safety and efficacy of galantamine in any illness occurring in children. Therefore, use of GALANTAMINE TABLETS USP in children is not recommended.
INTERACTIONS AND INCOMPATIBILITIES :
Use with Anticholinergics :
GALANTAMINE TABLETS USP has the potential to interfere with the activity of anticholinergic medications.
Use with Cholinomimetics and Other Cholinesterase Inhibitors :
A synergistic effect is expected when cholinesterase inhibitors are given concurrently with succinylcholine, other cholinesterase inhibitors, similar neuromuscular blocking agents or cholinergic agonists such as bethanechol.
A. Effect of Other Drugs on Galantamine :
In vitro :
CYP3A4 and CYP2D6 are the major enzymes involved in the metabolism of galantamine. CYP3A4 mediates the formation of galantamine-N-oxide; CYP2D6 leads to the formation of O-desmethyl-galantamine. Because galantamine is also glucuronidated and excreted unchanged, no single pathway appears predominant.
Cimetidine and Ranitidine :
Galantamine was administered as a single dose of 4 mg on day 2 of a 3-day treatment with either cimetidine (800 mg daily) or ranitidine (300 mg daily). Cimetidine increased the bioavailability of galantamine by approximately 16 %. Ranitidine had no effect on the PK of galantamine.
Ketoconazole :
Ketoconazole, a strong inhibitor of CYP3A4 and an inhibitor of CYP2D6, at a dose of 200 mg BID for 4 days, increased the AUC of galantamine by 30 %.
Erythromycin :
Erythromycin, a moderate inhibitor of CYP3A4, at a dose of 500 mg QID for 4 days, affected the AUC of galantamine minimally (10 % increase).
Paroxetine :
Paroxetine, a strong inhibitor of CYP2D6, at 20 mg/day for 16 days, increased the oral bioavailability of galantamine by about 40 %.
Memantine :
Memantine, an N-methyl-D-aspartate receptor antagonist, at a dose of 10 mg BID had no effect on the pharmacokinetics of galantamine (16 mg/day) at steady state.
B. Effect of Galantamine on Other Drugs :
Galantamine did not inhibit the metabolic pathways catalyzed by CYP1A2, CYP2A6, CYP3A4, CYP4A, CYP2C, CYP2D6 or CYP2E1. This indicates that the inhibitory potential of galantamine towards the major forms of cytochrme P450 is very low.
Warfarin :
Galantamine at 24 mg/day had no effect on the pharmacokinetics of R-and-S-warfarin (25 mg single dose) or on the prothrombin time. The protein binding of warfarin was unaffected by galantamine.
Digoxin :
Galantamine at 24 mg/day had no effect on the steady-state pharmacokinetics of digoxin (0.375 mg once daily) when they were co-administered. In this study, however, one healthy subject was hospitalized for 2nd and 3rd degree heart block and bradycardia.
OVERDOSAGE :
Symptoms :
Signs and symptoms of significant overdosing of galantamine are predicted to be similar to those of overdosing of other cholinomimetics. These effects generally involve the central nervous system, the parasympathetic nervous system, and the neuromuscular junction. In addition to muscle weakness or fasciculations, some or all of the signs of a cholinergic crisis may develop : severe nausea, vomiting, gastro-intestinal cramping, salivation, lacrimation, urination, defecation, sweating, bradycardia, hypotension, collapse and convulsions. Increasing muscle weakness together with tracheal hypersecretions and bronchospasm, may lead to vital airway compromise. There have been post-marketing reports of torsade de pointes, QT prolongation, bradycardia, ventricular tachycardia and brief loss of consciousness in association with inadvertent overdoses of galantamine. In one case where the dose was known, eight 4 mg tablets (32 mg total) were ingested on a single day. Two additional cases of accidental ingestion of 32 mg (nausea, vomiting, and dry mouth; nausea, vomiting, and substernal chest pain) and one of 40 mg (vomiting) resulted in brief hospitalisations for observation with full recovery. One patient, who was prescribed 24 mg/day and had a history of hallucinations over the previous two years, mistakenly received 24 mg twice daily for 34 days and developed hallucinations requiring hospitalisation. Another patient, who was prescribed 16 mg/day of oral solution, inadvertently ingested 160 mg (40 ml) and experienced sweating, vomiting, bradycardia, and near-syncope one hour later, which necessitated hospital treatment. His symptoms resolved within 24 hours.
TREATMENT OF OVERDOSAGE :
As in any case of overdose, general supportive measures should be used. In severe cases, anticholinergics such as atropine can be used as a general antidote for cholinomimetics. An initial dose of 0.5 to 1.0 mg intravenously is recommended, with subsequent doses
based on the clinical response.
STORAGE :
Store at controlled room temperature 15°-30°C (59°- 86°F), protected from moisture and light. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
GALANTAMINE TABLETS USP contains Galantamine Hydrobromide USP equivalent to Galantamine 8 mg. 1 Strip of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





