
25 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
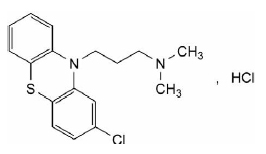
CHLORPROMAZINE HYDROCHLORIDE TABLETS USP (Chlorpromazine Hydrochloride) is a phenothiazine antipsychotic. Chemically, chlorpromazine hydrochloride is 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-,monohydrochloride. The molecular formula is C17H19ClN2S·HCland molecular weight is 355.33.
CHLORPROMAZINE HYDROCHLORIDE TABLETS USP are brown coloured, circular, biconvex film coated tablets having breakline on one side and “SGP” embossed on other side.
COMPOSITION :
Each film coated tablet contains :
Chlorpromazine Hydrochloride USP 25 mg
Excipients q.s.
Colours : Red Ferric Oxide NF, Yellow Ferric Oxide NF
ACTIONS :
Chlorpromazine Hydrochloride has depressant actions on the central nervous system, with alpha-adrenergic blocking and anti-cholinergic activities. It inhibits Dopamine and Prolactin release-inhibitory factor, thus stimulating the release of Prolactin. It increases the turnover of Dopamine in the brain. It has antiemetic, anti-pruritic, serotonin-blocking and weak antihistamine properties and slight ganglion blocking activity. It inhibits the heat regulating centre in the brain, and is analgesic and can relax skeletal muscle. Due to its action on the autonomic system it produces vasodilation, hypotension and tachycardia. Salivary and gastric secretions are reduced.
PHARMACOKINETICS :
Chlorpromazine Hydrochloride is readily, although sometimes erratically, absorbed from the gastrointestinal tract; peak plasma concentrations are attained 2 to 4 hours after ingestion. It is subject to considerable first-pass metabolism in the gut wall and is also extensively metabolised in the liver and is excreted in the urine and bile in the form of numerous active and inactive metabolites; there is some evidence of enterohepatic recycling. Owing to the first-pass effect, plasma concentrations after oral doses are much lower than those after intramuscular doses. Moreover, there is very wide inter subject variation in plasma concentrations of chlorpromazine hydrochloride; no simple correlation has been found between plasma concentrations of chlorpromazine hydrochloride and its metabolites, and their therapeutic effect. Paths of metabolism of chlorpromazine hydrochloride include hydroxylation and conjugation with glucuronic acid, N-oxidation, oxidation of a sulfur atom, and dealkylation. Although the plasma half-life of chlorpromazine hydrochloride itself has been reported to be about 30 hours, elimination of the metabolites may be very prolonged. There is limited evidence that chlorpromazine hydrochloride induces its own metabolism. Chlorpromazine Hydrochloride is about 95 to 98 % bound to plasma proteins. It is widely distributed in the body and crosses the blood-brain barrier to achieve higher concentrations in the brain than in the plasma. Chlorpromazine Hydrochloride and its metabolites also cross the placenta and are istributed into breast milk.
INDICATIONS :
1. For the management of manifestations of psychotic disorders.
2. For the treatment of schizophrenia.
3. To control nausea and vomiting.
4. For relief of restlessness and apprehension before surgery.
5. For acute intermittent porphyria.
6. As an adjunct in the treatment of tetanus.
7. To control the manifestations of the manic type of manicdepressive illness.
8. For relief of intractable hiccups.
For the treatment of severe behavioural problems in children (1 to 12 years of age) marked by combativeness and/or explosive hyperexcitable behaviour (out of proportion to immediate provocations), and in the short-term treatment of hyperactive children who show excessive motor activity with ccompanying conduct disorders consisting of some or all of the following symptoms : impulsivity, difficulty sustaining attention, aggressivity, mood lability and poor frustration tolerance.
Administration :
For oral use.
Dosage :
Dosages should be low to begin with and gradually increased under close supervision until the optimum dosage for the individual is reached. Individuals vary considerably and the optimum dose may be affected by the formulation used. Dosage of chlorpromazine hydrochloride in schizophrenia, other psychoses, anxiety and agitation etc.
Adult :
Initially 25 mg t.d.s. or 75 mg at bedtime increasing by daily amounts of 25 mg to an effective maintenance dose. This is usually in the range 75 to 300 mg daily but some patients may require up to 1 g daily.
Children under 1 year :
Do not use unless the risk benefit ratio has been assessed.
Children 1-5 years :0.5 mg/kg body weight every 4-6 hours to a maximum recommended dose of 40 mg daily.
Children 6-12 years :
⅓-½ adult dose to a maximum recommended dose of 75 mg daily.
Elderly or debilitated patients :
Start with ⅓-½ usual adult dose with a more gradual increase in dosage.
Hiccups :
Adult :
25-50 mg t.d.s. or q.d.s.
Children under 1 year :
No information available.
Children 1-5 years :
No information available.
Children 6-12 years :
No information available.
Elderly or debilitated patients :
As for adults.
Nausea and vomiting of terminal illness :
Adults :
10-25 mg every 4-6 hours.
Children under 1 year :
Do not use unless the risk-benefit ratio has been assessed.
Children 1-5 years :
0.5 mg/kg every 4-6 hours. Maximum daily dosage should not exceed 40 mg.
Children 6-12 years :
0.5 mg/kg every 4-6 hours. Maximum daily dosage should not exceed 75 mg.
Elderly or debilitated patients :
Initially ⅓-½ adult dose. The physician should then use his clinical judgment to obtain control.
CONTRAINDICATIONS :
CHLORPROMAZINE HYDROCHLORIDE TABLETS USP should be avoided in patients with liver or renal dysfunction, epilepsy, Parkinson’s disease, hypothyroidism, cardiac failure, phaeochromocytoma, myasthenia gravis, prostate hypertrophy. It should be avoided in patients known to be hypersensitive to phenothiazines or with a history of narrow angle glaucoma. Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.).
Increased Mortality in Elderly Patients with Dementia-Related Psychosis :
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Chlorpromazine Hydrochloride is not approved for the treatment of patients with dementia related psychosis (see Boxed Warning). The extrapyramidal symptoms which can occur secondary to chlorpromazine hydrochloride may be confused with the central nervous system signs of an undiagnosed primary disease responsible for the vomiting; e.g., Reye’s syndrome or other encephalopathy. The use of chlorpromazine hydrochloride and other potential hepatotoxins should be avoided in children and adolescents whose signs and symptoms suggest Reye’s syndrome.
Tardive Dyskinesia :
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses. There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and therapy may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and, 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically. If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
Blood Dyscrasias :
Agranulocytosis has been reported rarely, most commonly in the first three months of treatment, but occasionally later. Blood counts should be performed if the patient develops signs of a persistent infection. Transient leucopenia can also occur. Other blood dyscrasias including thrombocytopenia and haemolytic anaemia have occurred very rarely. Cases of venous thromboembolism (VTE) have been reported with antipsychotic drugs. Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible risk factors for VTE should be identified before and during treatment with CHLORPROMAZINE HYDROCHLORIDE TABLETS USP and preventive measures undertaken. Chlorpromazine Hydrochloride commonly causes increased susceptibility to sunburn and patients should be warned to avoid excessive exposure. Phototoxic or photoallergic reactions may occur. Various skin rashes and reactions, including exfoliative dermatitis and erythema multiforme have been reported. Contact skin sensitivity may be produced by contact with chlorpromazine hydrochloride. The occurrence of antinuclear antibodies has been reported. SLE has very rarely occurred. Chlorpromazine Hydrochloride impairs body temperature regulation and cases of severe hypothermia or hyperpyrexia have been reported, usually in association with moderate or high dosage. The elderly or hypothyroid patient may be particularly susceptible to hypothermia. The hazard of hyperthermia may be increased by especially hot or humid weather or by drugs, such as antiparkinson agents, which impair sweating. It has also been reported after intramuscular injections of chlorpromazine hydrochloride.
Chlorpromazine Hydrochloride can rarely cause obstructive jaundice associated with stasis in biliary canaliculi. It has been thought to be a hypersensitivity reaction and some cases have shown premonitory fever and associated eosinophilia. It has normally been reversible on stopping the drug, but extremely rare cases of progressive liver disease have been reported. In most cases the jaundice has appeared between one to four weeks after the start of the treatment. Chlorpromazine Hydrochloride treatment should be withdrawn and not given again. Transient abnormalities of liver function tests may occur in the absence of jaundice. Faecal impaction, severe paralytic ileus or megacolon have been reported. The signs of intestinal obstruction may be obscured by the antiemetic action of chlorpromazine hydrochloride. An approximately 3-fold increased risk of cerebrovascular adverse events has been seen in randomised placebo controlled clinical trials in the dementia population with some atypical antipsychotics. The mechanism for this increased risk is not known. An increased risk cannot be excluded for other antipsychotics or other patient populations. Chlorpromazine Hydrochloride should be used with caution in patients with risk factors for stroke. Chlorpromazine Hydrochloride should be used with caution in patients with cardiovascular disease or a family history of QT prolongation. Concomitant neuroleptics should also be avoided.
Long-Term Therapy :
To lessen the likelihood of adverse reactions related to cumulative drug effect, patients with a history of long-term therapy with chlorpromazine hydrochloride and/or other antipsychotics should be evaluated periodically to decide whether the maintenance dosage could be lowered or drug therapy iscontinued.
Antiemetic Effect :
The antiemetic action of chlorpromazine hydrochloride may mask the signs and symptoms of overdosage of other drugs and may obscure the diagnosis and treatment of other conditions such as intestinal obstruction, brain tumour and Reye’s syndrome. When chlorpromazine hydrochloride is used with cancer chemotherapeutic drugs, vomiting as a sign of the toxicity of these agents may be obscured by the antiemetic effect of chlorpromazine hydrochloride.
Abrupt Withdrawal :
Like other phenothiazines, chlorpromazine hydrochloride is not known to cause psychic dependence and does not produce tolerance or addiction. There may be, however, following abrupt withdrawal of high-dose therapy, some symptoms resembling those of physical dependence such as gastritis, nausea and vomiting, dizziness and tremulousness. These symptoms can usually be avoided or reduced by gradual reduction of the dosage or by continuing concomitant anti-parkinsonism agents for several weeks after chlorpromazine hydrochloride is withdrawn.
Pregnancy :
There is inadequate evidence of the safety of CHLORPROMAZINE HYDROCHLORIDE TABLETS USP in human pregnancy but it has been widely used for many years without apparent ill consequence. However, there is evidence of harmful effects in animals, so like other drugs, it should be avoided in pregnancy unless the physician considers it essential. It may occasionally prolong labour and at such a time should be withheld until the cervix is dilated 3-4 cm. Possible adverse effects on the foetus include lethargy or paradoxical hyper excitability, tremor and low Apgar score.
Nursing mothers :
There is evidence that chlorpromazine hydrochloride is excreted in the breast milk of nursing mothers. Because of the potential for serious adverse reactions in nursing infants from chlorpromazine hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
INTERACTIONS AND INCOMPATIBILITIES :
Interactions :
The CNS depressant actions of CHLORPROMAZINE HYDROCHLORIDE TABLETS USP and other neuroleptic agents may be intensified (additively) by alcohol, cimetidine, opioid, analgesics, barbiturates and other sedatives. Respiratory depression may occur. The hypotensive effect of most antihypertensive drugs especially alpha adrenoceptor blocking agents may be exaggerated by CHLORPROMAZINE HYDROCHLORIDE TABLETS USP. The mild anticholinergic effect of CHLORPROMAZINE HYDROCHLORIDE TABLETS USP may be enhanced by other anticholinergic drugs possibly leading to constipation, heat stroke etc. Phenothiazines enhance the hypotensive effect of anaesthetics and calcium channel blockers. Severe postural hypotension may occur with concomitant administration of chlorpromazine hydrochloride and ACE inhibitors. The action of some drugs may be opposed by CHLORPROMAZINE HYDROCHLORIDE TABLETS USP; these include amphetamine, levodopa, clonidine, guanethidine, adrenaline. Anticholinergic agents may reduce the antipsychotic effect of CHLORPROMAZINE HYDROCHLORIDE TABLETS USP. Some drugs interfere with absorption of neuroleptic agents; antacids, antiparkinson, lithium. Increases or decreases in the plasma concentrations of a number of drugs, e.g. propanolol, phenobarbitone have been observed but were not of clinical significance. Phenothiazines increase the risk of ventricular arrhythmias with drugs that prolong the QT interval such as sotalol. The serum concentration of chlorpromazine hydrochloride may be increased by some antimalarial agents. High doses of CHLORPROMAZINE HYDROCHLORIDE TABLETS USP reduce the response to hypoglycaemic agents, the dosage of which might have to be raised. Chlorpromazine Hydrochloride should not be taken with QT prolonging drugs, drugs causing electrolyte imbalance and metabolic inhibitors. Documented clinically significant adverse interactions occur with alcohol, guanethidine and hypoglycaemic agents. Adrenaline must not be used in patients overdosed with CHLORPROMAZINE HYDROCHLORIDE TABLETS USP. Other interactions are of a theoretical nature and not serious.
Cardiovascular :
Hypotensive Effects :
Postural hypotension, simple tachycardia, momentary fainting and dizziness may occur rarely, after the first oral dose. Usually recovery is spontaneous and symptoms disappear within 1/2 to hours. Occasionally, these effects may be more severe and prolonged, producing a shock-like condition. To control hypotension, place patient in head-low position with legs raised. If a vasoconstrictor is required, norepinephrine and phenylephrine are the most suitable. Other pressor agents, including epinephrine, should not be used as they may cause a paradoxical further lowering of blood pressure.
EKG Changes :
Particularly nonspecific, usually reversible Q and T wave distortions – have been observed in some patients receiving phenothiazine tranquilizers, including chlorpromazine hydrochloride. Note : Sudden death, apparently due to cardiac arrest, has been reported.
CNS Reactions :
Extrapyramidal Symptoms :
Neuromuscular reactions include dystonias, motor restlessness, pseudo-parkinsonism and tardive dyskinesia, and appear to be dose-related. They are discussed in the following paragraphs :
Dystonia :
Class effect : Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment.
Dystonic symptoms include :
spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Motor Restlessness :
Symptoms may include agitation or jitteriness and sometimes insomnia. These symptoms often disappear spontaneously. At times, these symptoms may be similar to the original neurotic or psychotic symptoms. Dosage should not be increased until these side effects have subsided. If these symptoms become too troublesome, they can usually be controlled by a reduction of dosage or change of drug. Treatment with anti-parkinsonian agents, benzodiazepines or propranolol may be helpful.
Pseudo-parkinsonism :
Symptoms may include : mask-like facies, drooling, tremors, pill rolling motion, cogwheel rigidity and shuffling gait. In most cases, these symptoms are readily controlled when an anti-parkinsonism agent is administered concomitantly. Anti-parkinsonism agents should be used only when required. Generally, therapy of a few weeks to 2 or 3 months will suffice. After this time patients should be evaluated to determine their need for continued treatment. (Note : Levodopa has not been found effective in antipsychoticinduced pseudo-parkinsonism.) Occasionally, it is necessary to lower the dosage of chlorpromazine hydrochloride or to discontinue the drug.
Tardive Dyskinesia :
As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The syndrome can also develop, although much less frequently, after relatively brief treatment periods at low doses. This syndrome appears in all age groups. Although its prevalence appears to be highest among elderly patients, especially elderly women, it is impossible to rely upon prevalence estimates to predict at the inception of antipsychotic treatment which patients are likely to develop the syndrome. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movements of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities. In rare instances, these involuntary movements of the extremities are the only manifestations of tardive dyskinesia. A variant of tardive dyskinesia, tardive dystonia, has also been described.
There is no known effective treatment for tardive dyskinesia; anti-parkinsonism agents do not alleviate the symptoms of this syndrome. If clinically feasible, it is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been reported that fine vermicular movements of the tongue may be an early sign of the syndrome and if the medication is stopped at that time, the syndrome may not develop.
Adverse Behavioural Effects :
Psychotic symptoms and catatonic-like states have been reported rarely.
Other CNS Effects :
Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Cerebral oedema has been reported. Convulsive seizures (petit mal and grand mal) have been reported, particularly in patients with EEG abnormalities or history of such disorders. Abnormality of the cerebrospinal fluid proteins has also been reported. Allergic Reactions of a mild urticarial type or photosensitivity are seen. Avoid undue exposure to sun. More severe reactions, including exfoliative dermatitis, have been reported occasionally. Contact dermatitis has been reported in nursing personnel; accordingly, the use of rubber gloves when administering chlorpromazine hydrochloride liquid or injectable is recommended.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Patients should be warned about drowsiness during the early days of treatment, and advised not to drive or operate machinery.
OVERDOSAGE :
Symptoms :
Primarily symptoms of central nervous system depression to the point of somnolence or coma. Hypotension and extrapyramidal symptoms. Other possible manifestations include agitation and restlessness, convulsions, fever, autonomic reactions such as dry mouth and ileus, EKG changes and cardiac arrhythmias.
TREATMENT OF OVERDOSAGE :
It is important to determine other medications taken by the patient since multiple drug therapy is common in overdosage situations. Treatment is essentially symptomatic and supportive. Early gastric lavage is helpful. Keep patient under observation and maintain an open airway, since involvement of the extrapyramidal mechanism may produce dysphagia and respiratory difficulty in severe overdosage. Do not attempt to induce emesis because a dystonic reaction of the head or neck may develop that could result in aspiration of vomitus. Extrapyramidal symptoms may be treated with anti-parkinsonism drugs, barbiturates, diphenhydramine hydrochloride. If administration of a stimulant is desirable, amphetamine, dextroamphetamine, or caffeine with sodium benzoate is recommended. Stimulants that may cause convulsions (e.g., picrotoxin or pentylenetetrazol) should be avoided. If hypotension occurs, the standard measures for managing circulatory shock should be initiated. If it is desirable to administer a vasoconstrictor, norepinephrine bitartrate and phenylephrine hydrochloride are most suitable. Other pressor agents, including epinephrine, are not recommended because phenothiazine derivatives may reverse the usual elevating action of these agents and cause a further lowering of blood pressure.
STORAGE :
Stored below 30°C (86°F), protected from moisture and light. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CHLORPROMAZINE HYDROCHLORIDE TABLETS USP contains Chlorpromazine Hydrochloride USP 25 mg. 10 Blisters of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





