
10 mg, 25 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
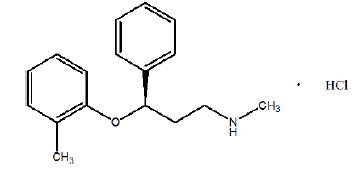
ATOMOXETINE CAPSULES USP (Atomoxetine Hydrochloride) is a selective norepinephrine reuptake inhibitor. Chemically, Atomoxetine is (-)-N-Methyl-3-phenyl-3-(o-tolyloxy) propylamine hydrochloride. The molecular formula is C17H21NO ·HCl and molecular weight is 291.82.
ATOMOXETINE CAPSULES USP are white or nearly white powder filled in hard gelatin capsules of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Atomoxetine Hydrochloride USP
equivalent to Atomoxetine 10 mg
Excipients q.s.
Approved colours used in empty capsule shells.
Each hard gelatin capsule contains :
Atomoxetine Hydrochloride USP
equivalent to Atomoxetine 18 mg
Excipients q.s.
Approved colours used in empty capsule shells.
Each hard gelatin capsule contains :
Atomoxetine Hydrochloride USP
equivalent to Atomoxetine 25 mg
Excipients q.s.
Approved colours used in empty capsule shells.
Each hard gelatin capsule contains :
Atomoxetine Hydrochloride USP
equivalent to Atomoxetine 40 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Atomoxetine is a highly selective and potent inhibitor of the pre-synaptic noradrenaline transporter, its presumed mechanism of action, without directly affecting the serotonin or dopamine transporters. Atomoxetine has minimal affinity for other noradrenergic receptors or for other neurotransmitter transporters or receptors. Atomoxetine has two major oxidative metabolites : 4-hydroxyatomoxetine and N-desmethylatomoxetine. 4-hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the noradrenaline transporter but, unlike atomoxetine, this metabolite also exerts some inhibitory activity at the serotonin transporter. However, any effect on this transporter is likely to be minimal, as the majority of 4-hydroxyatomoxetine is further metabolised such that it circulates in plasma at much lower concentrations (1 % of atomoxetine concentration in extensive metabolisers and 0.1 % of atomoxetine concentration in poor metabolisers). N-desmethylatomoxetine has substantially less pharmacological activity compared with atomoxetine. It circulates in plasma at lower concentrations in extensive metabolisers and at comparable concentrations to the parent drug in poor metabolisers at steady-state.
Atomoxetine is not a psychostimulant and is not an amphetamine derivative. In a randomised, double-blind, placebo-controlled, abuse-potential study in adults comparing effects of atomoxetine and placebo, atomoxetine was not associated with a pattern of response that suggested stimulant or euphoriant properties.
PHARMACOKINETICS :
Atomoxetine is rapidly absorbed after oral administration with absolute bioavailability of about 63 % in extensive metabolizers and 94 % in poor metabolizers. Maximal plasma concentrations (Cmax) are reached approximately 1 to 2 hours after dosing. Atomoxetine can be administered with or without food. Administration of atomoxetine with a standard high fat meal in adults did not affect the extent of oral absorption of atomoxetine (AUC) but did decrease the rate of absorption, resulting in a 37 % lower Cmax and delayed Tmax by 3 hours. In clinical trials with children and adolescents, administration of atomoxetine with food resulted in a 9 % lower Cmax. At therapeutic concentrations, 98 % of atomoxetine in plasma is bound to protein, primarily albumin. When doses were normalized to a mg/kg basis, similar half life, Cmax and AUC values were observed in adults, adolescents and children. Clearance and volume of distribution after adjustment for body weight were also similar.
Atomoxetine is eliminated primarily by oxidative metabolism through the CYP2D6 enzymatic pathway and subsequent glucuronidation. The major oxidative metabolite, regardless of CYP2D6 status, is 4-hydroxyatomoxetine, which is glucuronidated. 4-hydroxyatomoxetine is equipotent to atomoxetine as an inhibitor of the norepinephrine transporter, but circulates in plasma at much lower concentrations. Atomoxetine has a half life of 5 hours. A fraction of the population are poor metabolizers (of CYP2D6 metabolized drugs). These individuals have reduced activity in this pathway resulting in 10 fold higher AUCs, 5 fold higher peak plasma concentrations and slower elimination (plasma half life of 24 hours) of atomoxetine compared with people with normal activity extensive metabolizers.
Atomoxetine exposure (AUG) is increased, compared with normal subjects, in extensive metabolizer subjects with moderate and severe hepatic insufficiency. Extensive metabolizer subjects with end stage renal disease had higher systemic exposure to atomoxetine than healthy subjects (about 65 % increase), but there was no difference when exposure was corrected for mg/kg dose. The pharmacokinetics of atomoxetine has not been evaluated in the geriatric population. The pharmacokinetics of atomoxetine in children and adolescents are similar to those in adults. The pharmacokinetics of atomoxetine have not been evaluated in children under 6 years of age.
INDICATIONS :
ATOMOXETINE CAPSULES USP are indicated for the treatment of attention deficit hyperactivity disorder (initiated by a specialist physician experienced in managing the condition).
Administration :
ATOMOXETINE CAPSULES USP are for oral use only.
ATOMOXETINE CAPSULES USP may be administered with or without food. It can be discontinued without being tapered.
Dosage :
ADULT over 18 years, body-weight over 70 kg :
Initially 40 mg daily for 7 days, increased according to response; usual maintenance 80 – 100 mg daily, but may be increased to maximum 120 mg daily under the direction of a specialist.
CHILD 6–18 years, body-weight over 70 kg :
Initially 40 mg daily for 7 days, increased according to response; usual maintenance 80 mg daily, but may be increased to maximum 120 mg daily under the direction of a specialist.
ADULT and CHILD over 6 years, body-weight under 70 kg :
Initially 500 micrograms/kg daily for 7 days, increased according to response; usual maintenance 1.2 mg/kg daily, but may be increased to 1.8 mg/kg daily (maximum 120 mg daily) under the direction of a specialist.
Note :
Total daily dose may be given either as a single dose in the morning or in 2 divided doses with last dose no later than early evening.
CONTRAINDICATIONS :
ATOMOXETINE CAPSULES USP are contraindicated in patients known to have hypersensitivity to the active substance or any of its excipients.
Atomoxetine should not be used in combination with monoamine oxidase inhibitors (MAOI). ATOMOXETINE CAPSULES USP should not be used within a minimum of 2 weeks after discontinuing therapy with MAOI. Treatment with MAOI should not be initiated within 2 weeks after discontinuing atomoxetine. ATOMOXETINE CAPSULES USP was associated with an increased risk of mydriasis and therefore, its use is not recommended in patients with narrow-angle glaucoma. ATOMOXETINE CAPSULES USP should not be used in patients with severe cardiovascular or cerebrovascular disorders. Severe cardiovascular disorders may include severe hypertension, heart failure, arterial occlusive disease, haemodynamically significant congenital heart disease myocardial infarction, potentially life-threatening arrhythmias and channelopathies (disorders caused by the dysfunction of ion channels). Severe cerebrovascular disorders may include cerebral aneurysm or stroke. ATOMOXETINE CAPSULES USP should not be used in patients with phaeochromocytoma or a history of phaeochromocytoma.
Suicide-related behaviour :
Suicide-related behaviour (suicide attempts and suicidal ideation) has been reported in patients treated with atomoxetine. In double-blind clinical trials, suicide-related behaviours were uncommon, but more frequently observed among children and adolescents treated with atomoxetine compared to those treated with placebo, where there were no events. In adult double-blind clinical trials there was no difference in the frequency of suicide-related behaviour between atomoxetine and placebo. Patients who are being treated for ADHD should be carefully monitored for the appearance or worsening of suicide-related behaviour.
Sudden death and pre-existing cardiac abnormalities :
Sudden death has been reported in patients with structural cardiac abnormalities who were taking atomoxetine at usual doses. Although some serious structural cardiac abnormalities alone carry an increased risk of sudden death, atomoxetine should only be used with caution in patients with known serious structural cardiac abnormalities and in consultation with a cardiac specialist.
Cardiovascular effects :
Atomoxetine can affect heart rate and blood pressure. Most patients taking atomoxetine experience a modest increase in heart rate (mean < 10 bpm) and/or increase in blood pressure (mean < 5 mm Hg). However, combined data from controlled and uncontrolled ADHD clinical trials show that approximately 8-12 % of children and adolescents, and 6-10 % of adults experience more pronounced changes in heart rate (20 beats per minute or greater) and blood pressure (15-20 mmHg or greater). Analysis of these clinical trial data showed that approximately 15-26 % of children and adolescents, and 27-32 % of adults experiencing such changes in blood pressure and heart rate during atomoxetine treatment had sustained or progressive increases. Long-term sustained changes in blood pressure may potentially contribute to clinical consequences such as myocardial hypertrophy.
As a result of these findings, patients who are being considered for treatment with atomoxetine should have a careful history and physical exam to assess for the presence of cardiac disease, and should receive further specialist cardiac evaluation if initial findings suggest such history or disease. It is recommended that heart rate and blood pressure be measured and recorded before treatment is started and, during treatment, after each adjustment of dose and then at least every 6 months to detect possible clinically important increases. For paediatric patients the use of a centile chart is recommended. For adults, current reference guidelines for hypertension should be followed. Atomoxetine should not be used in patients with severe cardiovascular or cerebrovascular disorders. Atomoxetine should be used with caution in patients whose underlying medical conditions could be worsened by increases in blood pressure and heart rate, such as patients with hypertension, tachycardia, or cardiovascular or cerebrovascular disease.
Patients who develop symptoms such as palpitations, exertional chest pain, unexplained syncope, dyspnoea or other symptoms suggestive of cardiac disease during atomoxetine treatment should undergo a prompt specialist cardiac evaluation. In addition, atomoxetine should be used with caution in patients with congenital or acquired long QT or a family history of QT prolongation. As orthostatic hypotension has also been reported, atomoxetine should be used with caution in any condition that may predispose patients to hypotension or conditions associated with abrupt heart rate or blood pressure changes.
Cerebrovascular effects :
Patients with additional risk factors for cerebrovascular conditions (such as a history of cardiovascular disease, concomitant medications that elevate blood pressure) should be assessed at every visit for neurological signs and symptoms after initiating treatment with atomoxetine.
Hepatic effects :
Very rarely, spontaneous reports of liver injury, manifested by elevated hepatic enzymes and bilirubin with jaundice, have been reported. Also very rarely, severe liver injury, including acute liver failure, have been reported. ATOMOXETINE CAPSULES USP should be discontinued in patients with jaundice or laboratory evidence of liver injury, and should not be restarted.
Psychotic or manic symptoms :
Treatment-emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, mania or agitation in patients without a prior history of psychotic illness or mania can be caused by atomoxetine at usual doses. If such symptoms occur, consideration should be given to a possible causal role of atomoxetine, and discontinuation of treatment should be considered. The possibility that ATOMOXETINE CAPSULES USP will cause the exacerbation of pre-existing psychotic or manic symptoms cannot be excluded.
Pregnancy : Category C
No adequate and well controlled studies of atomoxetine have been done in pregnant women. Atomoxetine should not be used during pregnancy unless the potential benefit justifies the potential risk to the foetus.
Nursing mothers :
Atomoxetine and/or its metabolites were excreted in the milk of rats. It is not known if atomoxetine is excreted in human milk. Because of the lack of data, atomoxetine should be avoided during breast-feeding.
Paediatric Use :
The safety and efficacy of ATOMOXETINE CAPSULES USP in paediatric patients less than 6 years of age have not been established. The efficacy of ATOMOXETINE CAPSULES USP beyond 9 weeks and safety of ATOMOXETINE CAPSULES USP beyond 1 year of treatment have not been systematically evaluated. A study was conducted in young rats to evaluate the effects of atomoxetine on growth and neurobehavioural and sexual development. Rats were treated with 1, 10, or 50 mg/kg/day (approximately 0.2, 2, and 8 times, respectively, the maximum human dose on a mg/m2 basis) of atomoxetine given by gavage from the early postnatal period (Day 10 of age) through adulthood. Slight delays in onset of vaginal patency (all doses) and preputial separation (10 and 50 mg/kg), slight decreases in epididymal weight and sperm number (10 and 50 mg/kg), and a slight decrease in corpora lutea (50 mg/kg) were seen, but there were no effects on fertility or reproductive performance. A slight delay in onset of incisor eruption was seen at 50 mg/kg. A slight increase in motor activity was seen on Day 15 (males at 10 and 50 and females at 50 mg/kg) and on Day 30 (females at 50 mg/kg) but not on Day 60 of age. There were no effects on learning and memory tests.
The significance of these findings to humans is unknown.
INTERACTIONS :
MAOIs :
Atomoxetine should not be used with MAOIs.
CYP2D6 inhibitors (SSRIs (e.g., fluoxetine, paroxetine), quinidine, terbinafine) :
In patients receiving these drugs, atomoxetine exposure may be 6-to 8-fold increased and Css max 3 to 4 times higher, because it is metabolised by the CYP2D6 pathway. Slower titration and final lower dosage of atomoxetine may be necessary in patients who are already taking CYP2D6 inhibitor drugs. If a CYP2D6 inhibitor is prescribed or discontinued after titration to the appropriate atomoxetine dose has occurred, the clinical response and tolerability should be re-evaluated for that patient to determine if dose adjustment is needed.Caution is advised when combining atomoxetine with potent inhibitors of cytochrome P450 enzymes other than CYP2D6 in patients who are poor CYP2D6 metabolisers as the risk of clinically relevant increases in atomoxetine exposure in vivo is unknown.
Salbutamol (or other beta2 agonists) :
Atomoxetine should be administered with caution to patients treated with high dose nebulised or systemically administered salbutamol (or other beta2 agonists) because cardiovascular effects can be potentiated. Attention should be paid to monitoring heart rate and blood pressure, and dose adjustments may be justified for either atomoxetine or salbutamol (or other beta2 agonists) in the event of significant increases in heart rate and blood pressure during coadministration of these drugs. There is the potential for an increased risk of QT interval prolongation when atomoxetine is administered with other QT prolonging drugs (such as neuroleptics, class IA and III anti-arrhythmics, moxifloxacin, erythromycin, methadone, mefloquine, tricyclic antidepressants, lithium, or cisapride), drugs that cause electrolyte imbalance (such as thiazide diuretics), and drugs that inhibit CYP2D6. Seizures are a potential risk with atomoxetine. Caution is advised with concomitant use of medicinal drugs which are known to lower the seizure threshold (such as tricyclic antidepressants or SSRIs, neuroleptics, phenothiazines or butyrophenone, mefloquine, chloroquine, bupropion or tramadol). In addition, caution is advised when stopping concomitant treatment with benzodiazepines due to potential withdrawal seizures.
Anti-hypertensive drugs :
Atomoxetine should be used cautiously with anti-hypertensive drugs. Because of a possible increase in blood pressure, atomoxetine may decrease the effectiveness of anti-hypertensive drugs/drugs used to treat hypertension. Attention should be paid to monitoring of blood pressure and review of treatment of atomoxetine or anti-hypertensive drugs may be justified in the case of significant changes of blood pressure.
Pressor agents or drugs that increase blood pressure :
Because of possible increase in effects on blood pressure, atomoxetine should be used cautiously with pressor agents or medications that may increase blood pressure (such as salbutamol). Attention should be paid to monitoring of blood pressure, and review of treatment for either atomoxetine or pressor agents may be justified in the case of significant change in blood pressure.
Drugs that affect noradrenaline :
Drugs that affect noradrenaline should be used cautiously when co-administered with atomoxetine because of the potential for additive or synergistic pharmacological effects. Examples include antidepressants, such as imipramine, venlafaxine, and mirtazapine, or the decongestants pseudoephedrine or phenylephrine.
Drugs that affect gastric pH :
Drugs that elevate gastric pH (magnesium hydroxide/aluminium hydroxide, omeprazole) had no effect on atomoxetine bioavailability.
Drugs highly bound to plasma protein :
In vitro drug-displacement studies were conducted with atomoxetine and other highly-bound drugs at therapeutic concentrations. Warfarin, acetylsalicylic acid, phenytoin, or diazepam did not affect the binding of atomoxetine to human albumin. Similarly, atomoxetine did not affect the binding of these compounds to human albumin.
SIDE EFFECTS :
The most commonly reported adverse effects with atomoxetine are upper abdominal pain, constipation, dyspepsia, diarrhoea, dry mouth, nausea, vomiting, fatigue, mood swings, ear infection, influenza, decreased weight, decreased appetite, dizziness (vertigo), headache, somnolence, crying, irritability, cough, rhinorrhoea, dermatitis, palpitations, flatulence, pyrexia, rigors, sinusitis, myalgia, insomnia, paraesthesia, sinus headache, abnormal dreams decreased libido, sleep disorder, urinary hesitation and/or urinary retention and/or difficulty in micturition, dysmenorrhoea, ejaculation failure or ejaculation disorder, erectile disturbance, impotence, delayed menses, menstrual disorder, irregular menstruation, abnormal orgasm, prostatitis, dermatitis, increased sweating, hot flushes, mydriasis, pruritus, rash, peripheral coldness and lethargy.
EFFECTS ON ABILITY TO DRIVE AND USE
MACHINES :
Patients should be advised to use caution when driving a car or operating hazardous machinery until they are reasonably certain that their performance is not affected by atomoxetine.
INFORMATION FOR PATIENTS :
Patients should consult a physician if they are taking or plan to take any prescription or over-the counter medicines, dietary supplements, or herbal remedies. Patients should consult a physician if they are nursing, pregnant, or thinking of becoming pregnant while taking ATOMOXETINE CAPSULES USP. Patients may take ATOMOXETINE CAPSULES USP with or without food. If patients miss a dose, they should take it as soon as possible, but should not take more than the prescribed total daily amount of ATOMOXETINE CAPSULES USP in any 24-hours period.
OVERDOSAGE :
There is limited clinical trial experience with atomoxetine overdose and no fatalities were observed. During post marketing, there have been reports of non fatal acute and chronic overdoses of atomoxetine. The most commonly reported symptoms accompanying acute and chronic overdoses were somnolence, agitation, hyperactivity, abnormal behaviour and gastrointestinal symptoms. All events were mild to moderate. Signs and symptoms consistent with mild to moderate sympathetic nervous system activation (mydriasis, tachycardia, dry mouth) have also been observed. All patients recovered from these events.
TREATMENT OF OVERDOSAGE :
An airway should be established. Activated charcoal may be useful in limiting absorption if the patient presents within 1 hour of ingestion. Monitoring of cardiac and vital signs is recommended, along with appropriate symptomatic and supportive measures. The patient should be observed for a minimum of 6 hours. Because atomoxetine is highly protein-bound, dialysis is not likely to be useful in the treatment of overdose.
STORAGE :
Store at controlled room temperature 15°C - 30°C (59°F - 86°F), protected from moisture and light. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
ATOMOXETINE CAPSULES USP contain Atomoxetine Hydrochloride USP equivalent to Atomoxetine 10 mg/18 mg/25 mg/40 mg. 3 Blisters of 10 Capsules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





