
300 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
TRIENTINE HYDROCHLORIDE CAPSULES USP (Trientine Hydrochloride) is a copper chelator used in a similar way to penicillamine in the treatment of Wilson’s disease. It tends to be used in patients intolerant of penicillamine. Chemically, Trientine Hydrochloride is 1,2-Ethanediamine, N,N’-bis(2-aminoethyl)-, dihydrochloride. The molecular formula is C6H18N4 ·2HCl and molecular weight is 219.16.
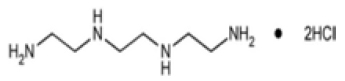
STRUCTURAL FORMULA :
Its structural formula is :
TRIENTINE HYDROCHLORIDE CAPSULES USP contains white to pale yellow crystalline hygroscopic powder filled in hard gelatin capsule of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Trientine Hydrochloride USP 300 mg
(equivalent to 200 mg base)
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Trientine hydrochloride is a copper-chelating agent which aids the elimination of copper from the body by forming a stable soluble complex that is readily excreted from the kidney.
PHARMACOKINETICS :
Data on the pharmacokinetics of trientine hydrochloride are not available.
INDICATIONS :
TRIENTINE HYDROCHLORIDE CAPSULES USP is indicated for the treatment of Wilson’s disease in patients intolerant of penicillamine.
Administration :
TRIENTINE HYDROCHLORIDE CAPSULES USP is for oral administration, preferably on an empty stomach. It is important that TRIENTINE HYDROCHLORIDE CAPSULES USP be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed.
Dosage :
Adult :
1.2 – 2.4 gm daily in 2 – 4 divided doses before food.
Child 2 – 12 years :
0.6 – 1.5 gm daily in 2 – 4 divided doses before food, adjusted according to response; reduce dose and increase frequency if nausea is a problem.
Child 12 – 18 years :
1.2 – 2.4 gm daily in 2 – 4 divided doses before food, adjusted according to response; reduce dose and increase frequency if nausea is a problem.
CONTRAINDICATION :
TRIENTINE HYDROCHLORIDE CAPSULES USP is contraindicated in patients with hypersensitivity to this product or components.
Patient experience with trientine hydrochloride is limited. Patients receiving TRIENTINE HYDROCHLORIDE CAPSULES USP should remain under regular medical supervision throughout the period of drug administration. Patients (especially women) should be closely monitored for evidence of iron deficiency anaemia.
PRECAUTIONS :
Trientine is not indicated as an alternative to D-Penicillamine in the treatment of rheumatoid arthritis or cystinuria. Penicillamine-induced systemic lupus erythematosus may not resolve on transfer to trientine. Trientine is a chelating agent which has been found to reduce serum iron levels possibly reducing its absorption. Iron supplementation may be necessary in some cases and should be administered at a 3 months after first opening at a different time of the day to trientine. There is no evidence that calcium or magnesium antacids
alter the efficacy of trientine but it is good practice to separate their administration (i.e. antacids should be taken after meals). There is no advantage in using trientine and penicillamine in combination.
There are no reports of hypersensitivity in patients who have been administered trientine hydrochloride for Wilson’s disease. However, there have been reports of asthma, bronchitis and dermatitis occurring after prolonged environmental exposure in workers who use trientine hydrochloride as a hardener of epoxy resins. Patients should be observed closely for signs of possible hypersensitivity.
Laboratory Test :
The most reliable index for monitoring treatment is the determination of free copper in the serum, which equals the difference between quantitatively determined total copper and ceruloplasmin-copper. Adequately treated patients will usually have less than 10 mcg free copper/dl of serum. Therapy may be monitored with a 24 hour urinary copper analysis periodically (i.e. every 6-12 months). Urine must be collected in copper-free glassware. Since a low copper diet should keep copper absorption down to less than one milligram a day, the patient probably will be in the desired state of negative copper balance if 0.5 to 1.0 milligram of copper is present in a 24-hour collection of urine.
Pregnancy : Category C
Trientine hydrochloride was teratogenic in rats at doses similar to the human dose. The frequencies of both resorptions and foetal abnormalities, including haemorrhage and oedema, increased while foetal copper levels decreased when trientine hydrochloride was given in the maternal diets of rats. There are no adequate and well-controlled studies in pregnant women. TRIENTINE HYDROCHLORIDE CAPSULES USP should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TRIENTINE HYDROCHLORIDE CAPSULES USP is administered to a nursing mother.
Paediatric use :
Controlled studies of the safety and effectiveness of TRIENTINE HYDROCHLORIDE CAPSULES USP in paediatric patients have not been conducted. It has been used clinically in paediatric patients as young as 6 years with no reported adverse experiences.
Geriatric Use :
Clinical studies of TRIENTINE HYDROCHLORIDE CAPSULES USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience is insufficient to determine differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
INTERACTIONS AND INCOMPATIBILITIES :
In general, mineral supplements should not be given since they may block the absorption of TRIENTINE HYDROCHLORIDE CAPSULES USP. However, iron deficiency may develop, especially in children and menstruating or pregnant women, or as a result of the low
copper diet recommended for Wilson’s disease. If necessary, iron may be given in short courses, but since iron and TRIENTINE HYDROCHLORIDE CAPSULES USP each inhibit absorption of the other, two hours should elapse between administration of TRIENTINE HYDROCHLORIDE CAPSULES USP and iron. It is important that TRIENTINE HYDROCHLORIDE CAPSULES USP be taken on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk.
This permits maximum absorption and reduces the likelihood of inactivation of the drug by metal binding in the gastrointestinal tract.
SIDE EFFECTS :
Trientine may cause nausea and skin rashes; duodenitis and colitis have also been reported. Iron deficiency may occur; if iron supplements are given an interval of at least 2 hours between the doses of trientine and iron has been recommended. Recurrence of symptoms of systemic lupus erythematosus has been reported in a patient who had previously reacted to penicillamine.
INFORMATION FOR PATIENTS :
Patients should be directed to take TRIENTINE HYDROCHLORIDE CAPSULES USP on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. Because of the potential for contact dermatitis, any site of exposure to the capsule contents should be washed with water promptly. For the first month of treatment, the patient should have his temperature taken nightly, and he should be asked to report any symptom such as fever or skin eruption.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
There is a report of an adult woman who ingested 30 grams of trientine hydrochloride without apparent ill effects. No other data on overdosage are available.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F), protected from moisture and light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
TRIENTINE HYDROCHLORIDE CAPSULES USP contains Trientine Hydrochloride 300 mg (equivalent to 200 mg base of Trientine).
3 Strips of 10 Capsules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





