
5 gm/20 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
AMINOCAPROIC ACID INJECTION (Aminocaproic Acid) is an antifibrinolytic used similarly to tranexamic acid in the treatment and prophylaxis of haemorrhage associated with excessive fibrinolysis. Chemically, Aminocaproic Acid is 6-Aminohexanoic acid. The molecular formula is C6H13NO2 and molecular weight is 131.17.
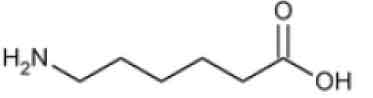
STRUCTURAL FORMULA :
Its structural formula is :
AMINOCAPROIC ACID INJECTION is a sterile, clear, colourless solution filled in vial of suitable size.
COMPOSITION :
Each ml contains :
Aminocaproic Acid USP 250 mg
Benzyl Alcohol B.P. 0.9 % v/v
(as preservative)
Water for Injection USP q.s.
ACTIONS :
Aminocaproic acid competitively inhibits activation of plasminogen, thereby reducing conversion of plasminogen to plasmin (fibrinolysin), an enzyme that degrades fibrin clots as well as fibrinogen and other plasma proteins including the procoagulant factors V and VIII.
Aminocaproic acid also directly inhibits plasmin activity, but higher doses are required than are needed to reduce plasmin formation. In vitro, the antifibrinolytic potency of aminocaproic acid is approximately one-fifth to one-tenth that of tranexamic acid.
PHARMACOKINETICS :
The fibrinolysis-inhibitory effects of Aminocaproic acid appear to be exerted principally via inhibition of plasminogen activators and to a lesser degree through antiplasmin activity. In adults, oral absorption appears to be a zero-order process with an absorption rate of 5.2 gm/hr. The mean lag time in absorption is 10 minutes. After a single oral dose of 5 gm, absorption was complete (F=1). Mean ± SD peak plasma concentrations (164 ± 28 mcg/ml) were reached within 1.2 ± 0.45 hours. After oral administration, the apparent volume of distribution was estimated to be 23.1 ± 6.6 l (mean ± SD). Correspondingly, the volume of distribution after intravenous administration has been reported to be 30.0 ± 8.2 l. After prolonged administration, Aminocaproic acid has been found to distribute throughout extravascular and intravascular compartments of the body, penetrating human red blood cells as well as other tissue cells.
Renal excretion is the primary route of elimination, whether Aminocaproic acid is administered orally or intravenously. Sixty-five percent of the dose is recovered in the urine as unchanged drug and 11 % of the dose appears as the metabolite adipic acid. Renal clearance (116 ml/min) approximates endogenous creatinine clearance. The total body clearance is 169 ml/min. The terminal elimination half-life for Aminocaproic acid is approximately 2 hours.
INDICATIONS :
AMINOCAPROIC ACID INJECTION is useful in enhancing haemostasis when fibrinolysis contributes to bleeding. In life-threatening situations, fresh whole blood transfusions, fibrinogen infusions, and other emergency measures may be required. Fibrinolytic bleeding may frequently be associated with surgical complications following heart surgery (with or without cardiac bypass procedures), and portacaval shunt; haematological disorders such as aplastic anaemia; acute and life-threatening abruption placentae; hepatic cirrhosis; and neoplastic disease such as carcinoma of the prostate, lung, stomach, and cervix. Urinary fibrinolysis, usually a normal physiological phenomenon, may frequently be associated with life-threatening complications following severe trauma, anoxia, and shock. Symptomatic of such complications is surgical haematuria (following prostatectomy and nephrectomy) or nonsurgical haematuria (accompanying polycystic or neoplastic diseases of the genitourinary system).
Administration :
For Intravenous Infusion.
DILUTE BEFORE USE.
AMINOCAPROIC ACID INJECTION is administered by infusion, utilizing the usual compatible intravenous vehicles (e.g., Sterile Water for Injection, Sodium Chloride for Injection, 5 % Dextrose or Ringer’s Injection). Although Sterile Water for Injection is compatible for intravenous injection the resultant solution is hypo-osmolar. RAPID INJECTION OF AMINOCAPROIC ACID INJECTION UNDILUTED INTO A VEIN IS NOT RECOMMENDED. Do not administer unless the solution is clear and seal is intact. Discard unused portion.
Dosage :
For the treatment of acute bleeding syndromes due to elevated fibrinolytic activity, it is suggested that 16 to 20 ml (4 to 5 gm) of aminocaproic acid in 250 ml of diluent be administered by infusion during the first hour of treatment, followed by a continuing infusion at the rate of 4 ml (1 gm) per hour in 50 ml of diluent. This method of treatment would ordinarily be continued for about 8 hours or until the bleeding situation has been controlled. Do not administer unless the solution is clear and seal is intact. Discard unused portion.
CONTRAINDICATIONS :
AMINOCAPROIC ACID INJECTION should not be used when there is evidence of an active intravascular clotting process. When there is uncertainty as to whether the cause of bleeding is primary fibrinolysis or disseminated intravascular coagulation (DIC), this distinction must be made before administering AMINOCAPROIC ACID INJECTION.
The following tests can be applied to differentiate the two conditions :
• Platelet count is usually decreased in DIC but normal in primary fibrinolysis.
• Protamine paracoagulation test is positive in DIC; a precipitate forms when protamine sulphate is dropped into citrated plasma. The test is negative in the presence of primary fibrinolysis.
• The euglobulin clot lysis test is abnormal in primary fibrinolysis but normal in DIC.
AMINOCAPROIC ACID INJECTION must not be used in the presence of DIC without concomitant heparin.
WARNINGS :
In patients with upper urinary tract bleeding, AMINOCAPROIC ACID INJECTION administration has been known to cause intrarenal obstruction in the form of glomerular capillary thrombosis or clots in the renal pelvis and ureters. For this reason, AMINOCAPROIC ACID INJECTION should not be used in haematuria of upper urinary tract origin, unless the possible benefits outweigh the risk. Subendocardial haemorrhages have been observed in dogs given intravenous infusions of 0.2 times the maximum human therapeutic dose of aminocaproic acid and in monkeys given 8 times the maximum human therapeutic dose of aminocaproic acid.
Fatty degeneration of the myocardium has been reported in dogs given intravenous doses of aminocaproic acid at 0.8 to 3.3 times the maximum human therapeutic dose and in monkeys given intravenous doses of aminocaproic acid at 6 times the maximum human therapeutic dose. Rarely, skeletal muscle weakness with necrosis of muscle fibers has been reported following prolonged administration. Clinical presentation may range from mild myalgias with weakness and fatigue to a severe proximal myopathy with rhabdomyolysis, myoglobinuria, and acute renal failure. Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy. AMINOCAPROIC ACID INJECTION administration should be stopped if a rise in CPK is noted. Resolution follows discontinuation of AMINOCAPROIC ACID INJECTION; however, the syndrome may recur if AMINOCAPROIC ACID INJECTION is restarted.
The possibility of cardiac muscle damage should also be considered when skeletal myopathy occurs. One case of cardiac and hepatic lesions observed in man has been reported. The patient received 2 gm of aminocaproic acid every 6 hours for a total dose of 26 gm. Death was due to continued cerebrovascular haemorrhage. Necrotic changes in the heart and liver were noted at autopsy. AMINOCAPROIC ACID INJECTION contains benzyl alcohol as preservative. Benzyl alcohol has been reported to be associated with a fatal “Gasping Syndrome” in premature infants. Symptoms include a striking onset of gasping syndrome, hypotension, bradycardia and cardiovascular collapse.
PRECAUTIONS :
General
AMINOCAPROIC ACID INJECTION, inhibits both the action of plasminogen activators and to a lesser degree, plasmin activity. The drug should NOT be administered without a definite diagnosis and/or laboratory finding indicative of hyperfibrinolysis (hyperplasminemia).
Rapid intravenous administration of the drug should be avoided since this may induce hypotension, bradycardia, and/or arrhythmia. Inhibition of fibrinolysis by aminocaproic acid may theoretically result in clotting or thrombosis. However, there is no definite evidence that administration of aminocaproic acid has been responsible for the few reported cases of intravascular clotting which followed this treatment. Rather, it appears that such intravascular clotting was most likely due to the patient’s pre-existing clinical condition, e.g., the presence of DIC. It has been postulated that extravascular clots formed in vivo may not undergo spontaneous lysis as do normal clots.
Pregnancy : Category C
Animal reproduction studies have not been conducted with aminocaproic acid. It is also not known whether aminocaproic acid can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. AMINOCAPROIC ACID INJECTION should be given to a pregnant woman only if clearly needed.
Nursing mothers :
It is not known whether aminocaproic acid is distributed into breast milk. However, problems in humans have not been documented.
Paediatric use :
Although studies on the relationship of age to the effects of aminocaproic acid have not been performed in the paediatric population, no paediatrics-specific problems attributed to aminocaproic acid have been documented to date. However, aminocaproic acid injections that contain benzyl alcohol should not be administered to premature neonates because the preservative has been associated with a fatal toxic syndrome consisting of metabolic acidosis, central nervous system (CNS) depression, respiratory problems, renal failure, hypotension, and possibly seizures and intracranial haemorrhages in these patients.
INTERACTIONS :
Prolongation of the template bleeding time has been reported during continuous intravenous infusion of AMINOCAPROIC ACID INJECTION at dosages exceeding 24 gm/day. Platelet function studies in these patients have not demonstrated any significant platelet dysfunction. However, in vitro studies have shown that at high concentrations (7.4 mMol/l or 0.97 mg/ml and greater) EACA inhibits ADP and collagen-induced platelet aggregation, the release of ATP and serotonin, and the binding of fibrinogen to the platelets in a concentration-response manner. Following a 10 gm bolus of AMINOCAPROIC ACID INJECTION, transient peak plasma concentrations of 4.6 mMol/l or 0.60 mg/ml have been obtained. The concentration of aminocaproic acid necessary to maintain inhibition of fibrinolysis is 0.99 mMol/l or 0.13 mg/ml. Administration of a 5 gm bolus followed by 1 to 1.25 gm/hr should achieve and sustain plasma levels of 0.13 mg/ml. Thus, concentrations which have been obtained in vivo clinically in patients with normal renal function are considerably lower than the in vitro concentrations found to induce abnormalities in platelet function tests. However, higher plasma concentrations of aminocaproic acid may occur in patients with severe renal failure.
SIDE EFFECTS :
AMINOCAPROIC ACID INJECTION is generally well tolerated. The following adverse experiences have been reported :
General : Oedema, headache, malaise.
Hypersensitivity Reactions : Allergic and anaphylactoid reactions, anaphylaxis.
Local Reactions : Injection site reactions, pain and necrosis.
Cardiovascular : Bradycardia, hypotension, peripheral ischemia, thrombosis.
Gastrointestinal : Abdominal pain, diarrhoea, nausea, vomiting.
Haematologic : Agranulocytosis, coagulation disorder, leucopenia, thrombocytopaenia.
Musculoskeletal : CPK increased, muscle weakness, myalgia, myopathy, myositis, rhabdomyolysis.
Neurologic : Confusion, convulsions, delirium, dizziness, hallucinations, intracranial hypertension, stroke, syncope.
Respiratory : Dyspnoea, nasal congestion, pulmonary embolism.
Skin : Pruritus, rash.
Special Senses : Tinnitus, vision decreased, watery eyes.
Urogenital : BUN increased, renal failure. There have been some reports of dry ejaculation during the period of Aminocaproic Acid Injection treatment. These have been reported to date only in haemophilia patients who received the drug after undergoing dental surgical procedures. However, this symptom resolved in all patients within 24 to 48 hours of completion of therapy.
INFORMATION FOR PATIENTS :
Caution patient to avoid sudden position changes to prevent orthostatic hypotension.
- Advise patient to use soft toothbrush or sponge for dental care.
- Instruct patient to report the following symptoms to health care provider : Gingival bleeding, epistaxis, haematuria, skin changes (e.g., ecchymosis, petechiae), difficulty in urination, reddish-brown urine, chest or leg pain, or breathing difficulty.
OVERDOSAGE :
A few cases of acute overdosage with AMINOCAPROIC ACID INJECTION administered intravenously have been reported. The effects have ranged from no reaction to transient hypotension to severe acute renal failure leading to death. One patient with a history of brain tumour and seizures experienced seizures after receiving an 8 gram bolus injection of AMINOCAPROIC ACID INJECTION. The single dose of AMINOCAPROIC ACID INJECTION causing symptoms of overdosage or considered to be life-threatening is unknown. Patients have tolerated doses as high as 100 grams while acute renal failure has been reported following a dose of 12 grams.
The intravenous and oral LD50 of aminocaproic acid were 3 and 12 gm/kg respectively, in the mouse and 3.2 and 16.4 gm/kg respectively in the rat. An intravenous infusion dose of 2.3 gm/kg was lethal in the dog. On intravenous administration, tonic-clonic convulsions were observed in dogs and mice.
TREATMENT OF OVERDOSAGE :
No treatment for overdosage is known, although evidence exists that aminocaproic acid is removed by haemodialysis and may be removed by peritoneal dialysis. Pharmacokinetic studies have shown that total body clearance of aminocaproic acid is markedly decreased in patients with severe renal failure.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C, protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
AMINOCAPROIC ACID INJECTION is supplied as 5 gm of Aminocaproic Acid USP in 20 ml vial.
Single vial of 20 ml is packed in a carton.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





