
50 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
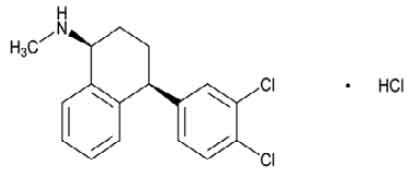
SERTRALINE HYDROCHLORIDE TABLETS USP (Sertraline Hydrochloride) is a antidepressant; antiobsessional; antipanic agent. Chemically, Sertraline Hydrochloride is (1S,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride. The molecular formula is C17H17Cl2N • HCl and molecular weight is 342.69.
STRUCTURAL FORMULA :
Its structural formula is :
SERTRALINE HYDROCHLORIDE TABLETS USP are blue coloured, elongated, biconvex film coated tablets with breakline on one side and other side plain.
COMPOSITION :
Each film coated tablet contains :
Sertraline Hydrochloride USP
equivalent to Sertraline 50 mg
Excipients q.s.
Colours : Aluminium Lake Indigo Carmine, Titanium Dioxide B.P.
ACTIONS :
Sertraline is a potent and selective inhibitor of neuronal uptake of serotonin (5-hydroxytryptamine [5-HT]). It has only weak effects on neuronal uptake of norepinephrine and dopamine. Chronic administration of sertraline in animals has resulted in down-regulation of postsynaptic beta-adrenergic receptors. Sertraline’s inhibition of serotonin reuptake enhances serotonergic transmission which results in subsequent inhibition of adrenergic activity in the locus ceruleus. Specifically, sertraline depresses the firing of the raphe serotonin neurons; this, in turn, increases the activity of the locus ceruleus, with consequent desensitization of the postsynaptic beta-receptors and presynaptic alpha 2-receptors. Sertraline lacks affinity for adrenergic (alpha1, alpha2, or beta) receptors, muscarinic-cholinergic receptors, gamma aminobutyric acid (GABA) receptors, dopaminergic receptors, histaminergic receptors, serotonergic (5-HT1A, 5-HT1B, 5-HT2) receptors, and benzodiazepine receptors. Sertraline does not inhibit monoamine oxidase.
Other actions/effects :
Sertraline inhibits the isoenzyme cytochrome P450 2D6 (CYP 2D6). When used in low clinical doses, sertraline probably inhibits CYP 2D6 less than other selective serotonin reuptake inhibitors. Sertraline blocks the uptake of serotonin into human platelets as well as into neurons. There have been rare reports of altered platelet function and of abnormal bleeding or purpura in patients taking sertraline. Sertraline has anorectic effects.
PHARMACOKINETICS :
Sertraline is slowly absorbed from the gastrointestinal tract with peak plasma concentrations occurring about 4.5 to 8.4 hours after ingestion. It undergoes extensive first-pass metabolism in the liver. The main pathway is demethylation to inactive N-desmethylsertraline, a process that appears to involve multiple cytochrome P450 isoenzymes; further metabolism and glucuronide conjugation occurs. Sertraline is widely distributed throughout body tissues and is about 98 % bound to plasma proteins. The plasma elimination half-life of sertraline is reported to be about 26 hours; steady-state concentrations are achieved after about one week with regular oral doses. Sertraline is excreted in about equal amounts in the urine and faeces, mainly as metabolites. Sertraline is distributed into breast milk. Bioavailability and absorption rate are increased if sertraline is taken with food.
INDICATIONS :
Adults :
SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of symptoms of depression, including depression accompanied by symptoms of anxiety, in patients with or without a history of mania. Following satisfactory response, continuation with SERTRALINE HYDROCHLORIDE TABLETS USP therapy is effective in preventing relapse of the initial episode of depression or recurrence of further depressive episodes. SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of obsessive compulsive disorder (OCD). Following initial response, sertraline has been associated with sustained efficacy, safety and tolerability in up 2 years of treatment of OCD. SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of panic disorder, with or without agoraphobia. SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of post-traumatic stress disorder (PTSD). SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of social phobia (social anxiety disorder). Following satisfactory response, continuation with sertraline therapy is effective in preventing relapse of the initial episode of social phobia. SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of premenstrual dysphoric disorder (PMDD).
Children and Adolescents (aged 6 to 17 years) :
SERTRALINE HYDROCHLORIDE TABLETS USP is indicated for the treatment of children and adolescents (aged 6 to 17 years) with OCD.
Administration :
SERTRALINE HYDROCHLORIDE TABLETS USP are for oral administration. SERTRALINE HYDROCHLORIDE TABLETS USP should be administered once daily with or without food, either in the morning or evening.
Dosage :
Initial Treatment in Adults :
Depression and OCD :
SERTRALINE HYDROCHLORIDE TABLETS USP treatment should be administered at a dose of 50 mg/day.
Panic Disorder, PTSD and Social Phobia :
Therapy for panic disorder, PTSD and social phobia should be initiated at 25 mg/day. After one week, the dose should be increased to 50 mg once daily. This dosage regimen has been shown to reduce the frequency of early treatment emergent side effects characteristic of panic disorder.
Premenstrual Dysphoric Disorder :
SERTRALINE HYDROCHLORIDE TABLETS USP treatment should be initiated with a dose of 50 mg/day, either daily throughout the menstrual cycle or limited to the luteal phase of the menstrual cycle, depending on physician assessment. Patients not responding to a 50 mg/day dose may benefit from dose increases (at 50 mg increments/menstrual cycle) up to 150 mg/day when dosing daily throughout the menstrual cycle, or 100 mg/day when dosing during the luteal phase of the menstrual cycle. If a 100 mg/day dose has been
established with luteal phase dosing, a 50 mg/day titration step for three days should be utilized at the beginning of each luteal phase dosing period. Dosage adjustments, which may include changes between dosage regimens (e.g., daily throughout the menstrual cycle versus during the luteal phase of the menstrual cycle), may be needed to maintain the patient on the lowest effective dosage and patients should be periodically reassessed to determine the need for continued treatment.
Titration in Adults :
For all Indications Other than PMDD :
Patients not responding to a 50 mg dose may benefit from dose increases. Dose changes should be made at intervals of at least one week, up to a maximum of 200 mg/day. The onset of therapeutic effect may be seen within 7 days. However, longer periods are usually necessary to demonstrate therapeutic response, especially in OCD.
Maintenance :
Dosage during long-term therapy should be kept at the lowest effective level, with subsequent adjustment depending on therapeutic response.
Use in Children and Adolescents (aged 6 to 17 years) :
The safety and efficacy of sertraline has been established in paediatric OCD patients aged 6 to 17. More than 250 paediatric OCD patients had been exposed to sertraline in completed and ongoing studies. The safety profile of sertraline in these paediatric studies is comparable to that observed in adult OCD studies. The administration of SERTRALINE HYDROCHLORIDE TABLETS USP to paediatric OCD patients (aged 13 to 17) should commence at 50 mg/day. Therapy for paediatric OCD patients (aged 6 to 12) should commence at 25 mg/day, increasing to 50 mg/day after one week. Subsequent doses may be increased in case of lack of response in 50 mg/day increments, up to 200 mg/day, as needed. In a clinical trial in patients aged 6 to 17 years with depression or OCD, sertraline appeared to have a similar pharmacokinetic profile to that found in adults. However, the generally lower body weights of children compared to those of adults should be taken into consideration in advancing the dose from 50 mg, in order to avoid excessive dosing.
WARNINGS AND PRECAUTIONS :
Serotonin Syndrome (SS) or Neuroleptic Malignant Syndrome (NMS) :
The development of potentially life-threatening syndromes like serotonin syndrome (SS) or Neuroleptic Malignant Syndrome (NMS) has been reported with SSRIs, including treatment with sertraline. The risk of SS or NMS with SSRIs is increased with concomitant use of serotonergic drugs (including triptans), with drugs which impair metabolism of serotonin (including MAOIs), antipsychotics and other dopamine antagonists. Patients should be monitored for the emergence of signs and symptoms of SS or NMS syndrome.
Switching from Selective Serotonin Reuptake Inhibitors (SSRIs), antidepressants or antiobsessional drugs :
There is limited controlled experience regarding the optimal timing of switching from SSRIs, antidepressants or anti-obsessional drugs to sertraline. Care and prudent medical judgment should be exercised when switching, particularly from long-acting agents such as fluoxetine.
Other serotonergic drugs e.g. tryptophan, fenfluramine and 5-HT agonists :
Co-administration of sertraline with other drugs which enhance the effects of serotonergic neurotransmission such as tryptophan or fenfluramine or 5-HT agonists, or the herbal medicine, St John’s Wort (hypericum perforatum), should be undertaken with caution and avoided whenever possible due to the potential for a pharmacodynamic interaction.
Activation of hypomania or mania :
Manic/hypomanic symptoms have been reported to emerge in a small proportion of patients treated with marketed antidepressant and anti-obsessional drugs, including sertraline. Therefore sertraline should be used with caution in patients with a history of
mania/hypomania. Close surveillance by the physician is required. Sertraline should be discontinued in any patient entering a manic phase.
Schizophrenia :
Psychotic symptoms might become aggravated in schizophrenic patients.
Seizures :
Seizures may occur with sertraline therapy : sertraline should be avoided in patients with unstable epilepsy and patients with controlled epilepsy should be carefully monitored. Sertraline should be discontinued in any patient who develops seizures.
Suicide/suicidal thoughts/suicide attempts or clinical worsening :
Depression is associated with an increased risk of suicidal thoughts, self harm and suicide (suicide-related events). This risk persists until significant remission occurs. As improvement may not occur during the first few weeks or more of treatment, patients should be closely monitored until such improvement occurs. It is general clinical experience that the risk of suicide may increase in the early stages of recovery. Other psychiatric conditions, for which sertraline is prescribed, can also be associated with an increased risk of suicide-related events. In addition, these conditions may be co-morbid with major depressive disorder. The same precautions observed when treating patients with major depressive disorder should therefore be observed when treating patients with other psychiatric disorders.
Patients with a history of suicide-related events, or those exhibiting a significant degree of suicidal ideation prior to commencement of treatment are known to be at greater risk of suicidal thoughts or suicide attempts, and should receive careful monitoring during treatment. A meta-analysis of placebo-controlled clinical trials of antidepressant drugs in adult patients with psychiatric disorders showed an increased risk of suicidal behaviour with antidepressants compared to placebo in patients less than 25 years old. Close supervision of patients and in particular those at high risk should accompany drug therapy especially in early treatment and following dose changes. Patients (and caregivers of patients) should be alerted about the need to monitor for any clinical worsening, suicidal behaviour or thoughts and unusual changes in behaviour and to seek medical advice immediately if these symptoms present.
Use in children and adolescents under 18 years of age :
SERTRALINE HYDROCHLORIDE TABLETS USP should not be used in the treatment of children and adolescents under the age of 18 years, except for patients with obsessive compulsive disorder aged 6-17 years old. Suicide-related behaviours (suicide attempt and suicidal thoughts), and hostility (predominantly aggression, oppositional behaviour and anger) were more frequently observed in clinical trials among children and adolescents treated with antidepressants compared to those treated with placebo. If, based on clinical need, a decision to treat is nevertheless taken; the patient should be carefully monitored for appearance of suicidal symptoms. In addition, long-term safety data in children and adolescents concerning growth, maturation and cognitive and behavioural development are lacking. Physicians must monitor paediatric patients on long term treatment for abnormalities in these body systems.
Abnormal bleeding/Haemorrhage :
There have been reports of cutaneous bleeding abnormalities such as ecchymoses and purpura and other hemorrhagic events such as gastrointestinal or gynaecological bleeding, with SSRIs. Caution is advised in patients taking SSRIs, particularly in concomitant use with drugs known to affect platelet function (e.g. anticoagulants, atypical antipsychotics and phenothiazines, most tricyclic antidepressants, acetylsalicylic acid and non-steroidal anti-inflammatory drugs (NSAIDs)) as well as in patients with a history of bleeding disorders.
Hyponatraemia :
Hyponatraemia may occur as a result of treatment with SSRIs or SNRIs including sertraline. In many cases, hyponatraemia appears to be the result of a syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases of serum sodium levels lower than 110 mmol/l have been reported. Elderly patients may be at greater risk of developing hyponatraemia with SSRIs and SNRIs. Also patients taking diuretics or who are otherwise volume-depleted may be at greater risk. Discontinuation of sertraline should be considered in patients with symptomatic hyponatraemia and appropriate medical intervention should be instituted. Signs and symptoms of hyponatraemia include headache, difficulty concentrating, memory impairment, confusion, weakness and unsteadiness which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Withdrawal symptoms seen on discontinuation of sertraline treatment :
Withdrawal symptoms when treatment is discontinued are common, particularly if discontinuation is abrupt. In one clinical trial, among patients treated with sertraline, the incidence of reported withdrawal reactions was 23 % in those discontinuing sertraline compared to 12 % in those who continued to receive sertraline treatment. The risk of withdrawal symptoms may be dependent on several factors including the duration and dose of therapy and the rate of dose reduction. Dizziness, sensory disturbances (including paraesthesia), sleep disturbances (including insomnia and intense dreams), agitation or anxiety, nausea and/or vomiting, tremor and headache are the most commonly reported reactions. Generally these symptoms are mild to moderate; however, in some patients they may be severe in intensity. They usually occur within the first few days of discontinuing treatment, but there have been very rare reports of such symptoms in patients who have inadvertently missed a dose. Generally these symptoms are self-limiting and usually resolve within 2 weeks, though in some individuals they may be prolonged (2 - 3 months or more). It is therefore advised that sertraline should be gradually tapered when discontinuing treatment over a period of several weeks or months, according to the patient’s needs.
Akathisia/psychomotor restlessness :
The use of sertraline has been associated with the development of akathisia, characterised by a subjectively unpleasant or distressing restlessness and need to move often accompanied by an inability to sit or stand still. This is most likely to occur within the first few weeks of treatment. In patients who develop these symptoms, increasing the dose may be detrimental.
Hepatic impairment :
Sertraline is extensively metabolised by the liver. A multiple dose pharmacokinetic study in subjects with mild, stable cirrhosis demonstrated a prolonged elimination half life and approximately three-fold greater AUC and Cmax in comparison to normal subjects. There were no significant differences in plasma protein binding observed between the two groups. The use of sertraline in patients with hepatic disease must be approached with caution. If sertraline is administered to patients with hepatic impairment, a lower or less frequent dose should be considered. Sertraline should not be used in patients with severe hepatic impairment.
Renal impairment :
Sertraline is extensively metabolised, and excretion of unchanged drug in urine is a minor route of elimination. In studies of patients with mild to moderate renal impairment (creatinine clearance 30 - 60 ml/min) or moderate to severe renal impairment (creatinine clearance 10 - 29 ml/min), multiple-dose pharmacokinetic parameters (AUC0-24 or Cmax) were not significantly different compared with controls. Sertraline dosing does not have to be adjusted based on the degree of renal impairment.
Diabetes :
In patients with diabetes, treatment with an SSRI may alter glycaemic control. Insulin and/or oral hypoglycaemic dosage may need to be adjusted.
St John’s Wort :
Concomitant use of the herbal remedy St John’s Wort (Hypericum perforatum) in patients receiving SSRIs should be avoided since there is a possibility of serotonergic potentiation.
Bone Fractures :
Epidemiological studies show an increased risk of bone fractures in patients receiving serotonin reuptake inhibitors (SRIs) including sertraline. The mechanism leading to this risk is not fully understood.
Electroconvulsive therapy :
There are no clinical studies establishing the risks or benefits of the combined use of ECT and sertraline.
Grapefruit juice :
The administration of SERTRALINE HYDROCHLORIDE TABLETS USP with grapefruit juice is not recommended.
Interference with urine screening tests :
False-positive urine immunoassay screening tests for benzodiazepines have been reported in patients taking sertraline. This is due to lack of specificity of the screening tests. False-positive test results may be expected for several days following discontinuation of sertraline therapy. Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish sertraline from benzodiazepines.
Angle-Closure Glaucoma :
SSRIs including sertraline may have an effect on pupil size resulting in mydriasis. This mydriatic effect has the potential to narrow the eye angle resulting in increased intraocular pressure and angle-closure glaucoma, especially in patients pre-disposed. SERTRALINE HYDROCHLORIDE TABLETS USP should therefore be used with caution in patients with angle-closure glaucoma or history of glaucoma.
Pregnancy : Category C
There are no well controlled studies in pregnant women. However, a substantial amount of data did not reveal evidence of induction of congenital malformations by sertraline. Animal studies showed evidence for effects on reproduction probably due to maternal toxicity caused by the pharmacodynamic action of the compound and/or direct pharmacodynamic action of the compound on the foetus. Use of sertraline during pregnancy has been reported to cause symptoms, compatible with withdrawal reactions, in some neonates, whose mothers had been on sertraline. This phenomenon has also been observed with other SSRI antidepressants. Sertraline is not recommended in pregnancy, unless the clinical condition of the woman is such that the benefit of the treatment is expected to outweigh the potential risk. Neonates should be observed if maternal use of sertraline continues into the later stages of pregnancy, particularly the third trimester. The following symptoms may occur in the neonate after maternal sertraline use in later stages of pregnancy : respiratory
distress, cyanosis, apnoea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycaemia, hypertonia, hypotonia, hyperreflexia, tremor, jitteriness, irritability, lethargy, constant crying, somnolence and difficulty in sleeping. These symptoms could be due to either serotonergic effects or withdrawal symptoms. In a majority of instances the complications begin immediately or soon (< 24 hours) after delivery. Epidemiological data have suggested that the use of SSRIs in pregnancy, particular in late pregnancy, may increase the risk of persistent pulmonary hypertension in the newborn (PPHN). The observed risk was approximately 5 cases per 1000 pregnancies. In the general population 1 to 2 cases of PPHN per 1000 pregnancies occur.
Nursing Mothers :
Sertraline is distributed into breast milk. Very low levels of sertraline and/or N-desmethylsertraline (< 2 nanograms/ml) were detected in the plasma of breast-fed infants of mothers who were receiving sertraline. However, no adverse effects in the infants were seen during the short-term (< 2 years) follow-up.
Paediatric Use :
Sertraline has been tested in children 6 to 17 years of age and, in effective doses, has not been shown to cause different side effects or problems than it does in adults. However, the effects of long-term use of sertraline on the growth, development, and maturation of
children and adolescents are unknown. Because of the anorectic effect of sertraline, body weight and growth should be monitored in children receiving long-term treatment.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Clinical pharmacology studies have shown that sertraline has no effect on psychomotor performance. However, as psychotropic drugs may impair the mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery, the patient should be cautioned accordingly.
INTERACTIONS AND INCOMPATIBILITIES :
Irreversible MAOIs (e.g. selegiline) :
SERTRALINE HYDROCHLORIDE TABLETS USP must not be used in combination with irreversible MAOIs such as selegiline. SERTRALINE HYDROCHLORIDE TABLETS USP must not be initiated for at least 14 days after discontinuation of treatment with an irreversible MAOI. SERTRALINE HYDROCHLORIDE TABLETS USP must be discontinued for at least 7 days before starting treatment with an irreversible MAOI.
Reversible, selective MAO-A inhibitor (moclobemide) :
Due to the risk of serotonin syndrome, the combination of sertraline with a reversible and selective MAOI, such as moclobemide, should not be given. Following treatment with a reversible MAO-inhibitor, a shorter withdrawal period than 14 days may be used before initiation of sertraline treatment. It is recommended that SERTRALINE HYDROCHLORIDE TABLETS USP should be discontinued for at least 7 days before starting treatment with a reversible MAOI.
Reversible, non-selective MAOI (linezolid) :
The antibiotic linezolid is a weak reversible and non-selective MAOI and should not be given to patients treated with sertraline. Severe adverse reactions have been reported in patients who have recently been discontinued from an MAOI and started on sertraline, or have recently had sertraline therapy discontinued prior to initiation of an MAOI. These reactions have included tremor, myoclonus, diaphoresis, nausea, vomiting, flushing, dizziness, and hyperthermia with features resembling neuroleptic malignant syndrome, seizures, and death.
Pimozide :
Increased pimozide levels of approximately 35 % have been demonstrated in a study of a single low dose pimozide (2 mg). These increased levels were not associated with any changes in EKG. While the mechanism of this interaction is unknown, due to the narrow therapeutic index of pimozide, concomitant administration of sertraline and pimozide is contraindicated.
Co-administration with sertraline is not recommended :
CNS depressants and alcohol :
The co-administration of sertraline 200 mg daily did not potentiate the effects of alcohol, carbamazepine, haloperidol, or phenytoin on cognitive and psychomotor performance in healthy subjects; however, the concomitant use of sertraline and alcohol is not recommended.
Astemizole :
Sertraline inhibits cytochrome P450 enzymes and may increase the plasma concentrations of these medications, thereby increasing the risk of cardiac arrhythmias, concomitant use is not recommended.
Other serotonergic drugs :
Caution is also advised with fentanyl used in general anaesthesia or in the treatment of chronic pain.
Special Precautions :
Lithium :
In a placebo-controlled trial in normal volunteers, the co-administration of sertraline with lithium did not significantly alter lithium pharmacokinetics, but did result in an increase in tremor relative to placebo, indicating a possible pharmacodynamic interaction. When co-administering sertraline with lithium, patients should be appropriately monitored.
Phenytoin :
A placebo-controlled trial in normal volunteers suggests that chronic administration of sertraline 200 mg/day does not produce clinically important inhibition of phenytoin metabolism. Nonetheless, as some case reports have emerged of high phenytoin exposure in patients using sertraline, it is recommended that plasma phenytoin concentrations be monitored following initiation of sertraline therapy, with appropriate adjustments to the phenytoin dose. In addition, co-administration of phenytoin may cause a reduction of sertraline plasma levels. It cannot be excluded that other CYP 3A4 inducers, e.g. phenobarbital, carbamazepine, St John´s Wort, rifampicin may cause a reduction of sertraline plasma levels.
Triptans :
There have been rare post-marketing reports describing patients with weakness, hyperreflexia, incoordination, confusion, anxiety and agitation following the use ofsertraline and sumatriptan. Symptoms of serotonergic syndrome may also occur with other products of the same class (triptans). If concomitant treatment with sertraline and triptans is clinically warranted, appropriate observation of the patient is advised.
Warfarin :
Co-administration of sertraline 200 mg daily with warfarin resulted in a small but statistically significant increase in prothrombin time, which may in some rare cases unbalance the INR value. Accordingly, prothrombin time should be carefully monitored when sertraline
therapy is initiated or stopped.
Other drug interactions, digoxin, atenolol, cimetidine :
Co-administration with cimetidine caused a substantial decrease in sertraline clearance. The clinical significance of these changes is unknown. Sertraline had no effect on the beta-adrenergic blocking ability of atenolol. No interaction of sertraline 200 mg daily was
observed with digoxin.
Drugs affecting platelet function :
The risk of bleeding may be increased when medicines acting on platelet function (e.g. NSAIDs, acetylsalicylic acid and ticlopidine) or other medicines that might increase bleeding risk are concomitantly administered with SSRIs, including sertraline.
Drugs Metabolized by Cytochrome P450 :
Sertraline may act as a mild-moderate inhibitor of CYP 2D6. Chronic dosing with sertraline 50 mg daily showed moderate elevation (mean 23 % - 37 %) of steady-state desipramine plasma levels (a marker of CYP 2D6 isozyme activity). Clinical relevant interactions may occur with other CYP 2D6 substrates with a narrow therapeutic index like class 1C antiarrhythmics such as propafenone and flecainide, TCAs and typical antipsychotics, especially at higher sertraline dose levels. Sertraline does not act as an inhibitor of CYP 3A4, CYP 2C9, CYP 2C19, and CYP 1A2 to a clinically significant degree. This has been confirmed by in-vivo interaction studies with CYP 3A4 substrates (endogenous cortisol, carbamazepine, terfenadine, alprazolam), CYP 2C19 substrate diazepam, and CYP 2C9 substrates
tolbutamide, glibenclamide and phenytoin. In vitro studies indicate that sertraline has little or no potential to inhibit CYP 1A2.
Intake of three glasses of grapefruit juice daily increased the sertraline plasma levels by approximately 100 % in a cross-over study in eight Japanese healthy subjects. Therefore, the intake of grapefruit juice should be avoided during treatment with sertraline. Based on the interaction study with grapefruit juice, it cannot be excluded that the concomitant administration of sertraline and potent CYP 3A4 inhibitors, e.g. protease inhibitors, ketoconazole, itraconazole, posaconazole, voriconazole, clarithromycin, telithromycin and nefazodone, would result in even larger increases in exposure of sertraline. This also concerns moderate CYP 3A4 inhibitors, e.g. aprepitant, erythromycin, fluconazole, verapamil and diltiazem. The intake of potent CYP 3A4 inhibitors should be avoided during treatment with sertraline. Sertraline plasma levels are enhanced by about 50 % in poor metabolizers of CYP 2C19 compared to rapid metabolizers. Interaction with strong inhibitors of CYP 2C19, e.g. omeprazole, lansoprazole, pantoprazole, rabeprazole, fluoxetine, fluvoxamine cannot be excluded.
Incidence less frequent or rare :

 Cardiovascular
Cardiovascular





