
200 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
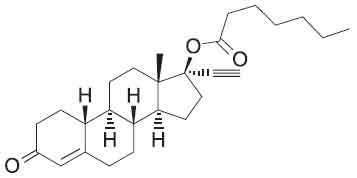
NORETHISTERONE ENANTHATE INJECTION (Norethisterone Enanthate) is a progestogen derived from nortestosterone that have weak oestrogenic and androgenic properties. Chemically, Norethisterone Enanthate is 17alpha-Ethynyl-19-nortestosterone 17-eptanoate.
The molecular formula is C27H38O3 and molecular weight is 410.59.
STRUCTURAL FORMULA :
Its structural formula is :
NORETHISTERONE ENANTHATE INJECTION is a sterile, pale yellow oily solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Norethisterone Enanthate 100 mg
Oily base q.s.
Contains no preservatives.
Each ml contains :
Norethisterone Enanthate 200 mg
Oily base q.s.
Contains no preservatives.
ACTIONS :
Progestogens prevent follicular maturation and rupture via a central mechanism. This also applies to norethisterone provided that it is present at a certain plasma level such as that achieved with norethisterone enanthate over 5 to 7 weeks after the injection. When
norethisterone enanthate is injected according to instructions, conception cannot take place during this period because no ovum is available to be fertilized. The cervical mucus is under the influence of the progestogen throughout the entire injection interval. Although motile spermatozoa can still be demonstrated in the cervical canal, they very rarely ascend as far as the uterus up to the 12th week of the injection interval. Under the influence of norethisterone enanthate, the lining of the endometrium is thinner, so fertilised eggs can’t attach themselves to it.
PHARMACOKINETICS :
Norethisterone enanthate is completely absorbed after intramuscular injection. The ester is quickly and eventually completely hydrolyzed to its pharmacologically active compound norethisterone once it is released from the depot. Maximum levels of norethisterone are measured about 3-20 days after intramuscular administration. They amount to average 13.4 ± 5.4 ng/ml and 12.2 ± 2.7 ng/ml about 7 days (median) after intramuscular administration of 200 mg norethisterone enanthate in 2 ml and 1 ml oily solution, respectively. Plasma levels of norethisterone declines in two disposition phases with half-lives of 4-5 days and 15-20 days, respectively, which are due to a biphasical release of norethisterone enanthate from the depot. Norethisterone enanthate is metabolized completely. Norethisterone enanthate is split mainly in the liver by enzymatic hydrolysis into norethisterone and heptanoic acid. While the fatty acid is metabolized by means of β-oxidation, norethisterone is transformed mainly through the reduction of the C4-C5 double bond and the C3 keto group.
The majority of metabolites found in urine are present as conjugates, mainly as sulfates, which are expected to be inactive. Qualitative transformation of norethisterone to ethinyloestradiol in vivo has been reported, but the contribution of this metabolic pathway to the pharmacological action of norethisterone is still unknown.
Up to 85 % of the norethisterone enanthate dose are excreted within 30 days in urine (40 %) and faeces (60 %). No unchanged norethisterone enanthate is recovered in urine or faeces. In urine and faeces, similar excretion half-lives of 6 - 9 days are estimated for
radioactive labelled substances during the observation period of 30 days and -in a further study - an excretion half-life of 20 - 30 days was measured in urine between day 30 and 80 after intramuscular administration of 200 mg 3H-norethisteterone enanthate. Based on animal studies, retention of the drug in the body is not to be expected. In plasma of women, 96 % of norethisterone are bound to proteins. The respective percentages bound to SHBG and albumin are approximately 35 % and 61 % as long as SHBG levels are within the normal range. Due to the half-life of the terminal disposition phase from plasma (about 2.5 weeks) and the initial dose regimen (one injection every 2 months), a slight accumulation of the drug will be expected after multiple administrations. A steady state will already be reached after the second administration. Transfer of norethisterone into mother’s milk is negligible. During the first week after intramuscular injection of norethisterone enanthate, a daily intake of norethisterone with mother’s milk in the range of 0.5 µg to 2.4 µg is
calculated from norethisterone concentrates in the milk, assuming that the infant ingests 600 ml milk daily. Although there is no direct investigation on bioavailability of norethisterone after intramuscular administration of norethisterone enanthate reported, complete availability can be estimated by comparison of norethisterone AUC values determined in different studies after intravenous injection of norethisterone and after intramuscular injection of norethisterone enanthate.
INDICATIONS :
NORETHISTERONE ENANTHATE INJECTION is indicated for injectable hormonal contraception. NORETHISTERONE ENANTHATE INJECTION is particularly suitable for women who cannot take oral contraceptives regularly or who do not tolerate them well.
NORETHISTERONE ENANTHATE INJECTION is intended for short-term use when a high level of efficacy independent of possible errors by the patient is required. It has been licensed for short-term use by women whose partners undergo vasectomy, until the vasectomy is effective, and women immunised against rubella, to prevent pregnancy during the period of activity of the virus. NORETHISTERONE ENANTHATE INJECTION can generally be used immediately after delivery or abortion.
Administration :
NORETHISTERONE ENANTHATE INJECTION is for deep intramuscular use given very slowly into gluteal muscle. It is advisable to place a plaster over the injection site after the injection to prevent any reflux of the NORETHISTERONE ENANTHATE INJECTION solution.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
NORETHISTERONE ENANTHATE INJECTION should only be administered to women with a history of normal cycles. Before starting the NORETHISTERONE ENANTHATE INJECTION, a through general medical and gynaecological examination (including the breasts) should be carried out and pregnancy must be excluded. The first intramuscular injection of 200 mg is given within the first 5 days of a cycle. The next three injections of 200 mg each are to be given at intervals of 8 weeks, after which a further injection of 200 mg is required every 12 weeks (84 days). If the injection interval is extended beyond this, no adequate contraceptive cover is available from the 13th week onwards. Should particular circumstances demand it (e.g. holidays, journeys), the interval between the injections can be shortened by 1 week. In each case, the next injection of 200 mg should only be given if a menstruation-like bleeding has occurred within the preceding 10 weeks. If it has not, the NORETHISTERONE ENANTHATE INJECTION must be discontinued and pregnancy excluded.
CONTRAINDICATIONS :
NORETHISTERONE ENANTHATE INJECTION is contraindicated in case of :
- Known or suspected pregnancy.
- Current or severe hepatic disease as long as liver function values have not returned to normal.
- Previous or existing liver tumours.
- Severe diabetes with vascular changes.
- Pathologically increased blood pressure.
- Disorders causing blood clots in the blood vessels (thromboembolic disorders).
- Disturbances of lipid metabolism.
- Suspected, existing or treated breast or endometrial cancer.
- Porphyria.
- Hypersensitivity to any of the components of Norethiesterone Enanthate.
- Abnormal vaginal bleeding due to unknown cause(s).
- Anaemia caused by a hereditary blood disorder when abnormal haemoglobin is produced (sickle cell anaemia).
- Metabolic disorder that runs in families and causes mild jaundice (Dublin-Johnson syndrome).
Medical Examination :
Assessment of women prior to starting NORETHISTERONE ENANTHATE INJECTION (and at regular intervals thereafter) should include a personal and family medical history of each woman. Physical examination should be guided by this and by the contraindications and warnings for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination including cervical cytology. Before starting treatment, pregnancy must be excluded. Women should be advised that progestogen-only contraceptives do not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Gynaecological considerations :
NORETHISTERONE ENANTHATE INJECTION should not be used in patients with abnormal uterine bleeding until a definite diagnosis has been established and the possibility of genital tract malignancy eliminated. Undiagnosed vaginal bleeding that is suspicious for
underlying conditions should be investigated prior to first injection and endometrial pathology should be excluded if it occurs in women over 40 after prolonged amenorrhoea. If there is a history of ectopic pregnancy or one fallopian tube is missing, the use of NORETHISTERONE ENANTHATE INJECTION should be decided on only after carefully weighing the benefits against the risks. If obscure lower abdominal complaints occur together with an irregular cycle pattern (above all amenorrhoea followed by persistent irregular bleeding), an extrauterine pregnancy must be considered. The patient should be informed before starting NORETHISTERONE ENANTHATE INJECTION that her menstrual pattern is likely to alter during the entire exposure period. Menstrual changes in the form of spotting, breakthrough bleeding and delayed menstruation are relatively frequent, and generally do not require treatment.
Amenorrhoea :
If, when the second injection is due, bleeding has not occurred in the preceding eight weeks the second injection should not be given until pregnancy has been ruled out.
Circulatory disorders :
There is a general opinion, based on statistical evidence, that users of hormonal contraceptives experience, more often than non-users, venous thromboembolism, arterial thrombosis, including cerebral and myocardial infarction, and subarachnoid haemorrhage. Full recovery from such disorders does not always occur, and it should be realised that in a few cases they are fatal. The relative risk of arterial thromboses (e.g. stroke and myocardial infarction) appears to increase further when heavy smoking, increasing age and the use of hormonal contraceptives coincide. Although there have been so far no observations of thromboembolic disease during the use of NORETHISTERONE ENANTHATE INJECTION, as a precaution it is recommended that this preparation should not be used where there is a history of thromboembolic processes.
No further injection should be given if symptoms of an arterial or venous thrombotic event occur during treatment, e.g.
- new onset or exacerbation of migraine-type headaches,
- sudden disturbances of vision or hearing perceptual disorders occur,
- significant rise in blood pressure,
- first signs of thrombosis or blood clots.
Liver Function :
Porphyria and existing impairment of liver function might theoretically be exacerbated by NORETHISTERONE ENANTHATE INJECTION. In rare cases benign, and in even rarer cases malignant, liver tumours leading in isolated cases to life-threatening intraabdominal haemorrhage, have been observed after the use of hormonal substances such as the one contained in NORETHISTERONE ENANTHATE INJECTION. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal haemorrhage occur, a liver tumour should be considered in the differential diagnosis. Women with a history of disturbed liver function or any disease that is prone to worsen during pregnancy such as idiopathic jaundice or severe pruritus of pregnancy should be carefully observed during medication. Recurrence of cholestatic jaundice which occurred first during pregnancy or previous use of sex steroids necessitates the discontinuation of NORETHISTERONE ENANTHATE INJECTION.
Other conditions :
A reduction of glucose tolerance has been observed in some women using progestogens. Consequently, diabetics and women with a tendency to diabetes should be carefully supervised during the use of NORETHISTERONE ENANTHATE INJECTION. In the case of diabetes, it may be necessary to reassess the required doses of antidiabetics or insulin. In rare cases coughing, dyspnoea and circulatory irregularities may occur during or immediately after the injection. Experience has shown that these reactions can be avoided by injecting NORETHISTERONE ENANTHATE INJECTION very slowly. Women with a history of severe depressive states should be carefully observed during medication. No further injection should be given, if during treatment, recurrence of earlier depression is experienced.
Effect on blood chemistry :
No influence of NORETHISTERONE ENANTHATE INJECTION on basal plasma cortisol, the ACTH test or the metyrapone test has been observed. In the acute dexamethasone suppression test, however, a higher plasma cortisol value than expected was found in
4 out of 10 women, although there were no clinical indications of disturbed adrenocortical function. A shortening of the recalcification time and of the thromboplastin time (Quick’s test) were observed in studies of the blood coagulation system.
Pregnancy : Category X
NORETHISTERONE ENANTHATE INJECTION is contraindicated in pregnancy. Like all nortestosterone derivatives used for contraception, NORETHISTERONE ENANTHATE INJECTION has slight androgenic activity, and a virilising effect on the external genitalia of a female foetus exposed to NORETHISTERONE ENANTHATE INJECTION after the first month of pregnancy cannot be totally ruled out on theoretical grounds. However, no such virilisation has been observed after the few pregnancies that have been reported during the use of NORETHISTERONE ENANTHATE INJECTION.
Nursing mothers :
There appear to be no adverse effects on infant growth or development when using NORETHISTERONE ENANTHATE INJECTION after six weeks postpartum. NORETHISTERONE ENANTHATE INJECTION does not appear to affect the quantity or quality of breast milk, however, minute amounts of the active substance are excreted with the milk and although considered harmless to a healthy neonate, might theoretically, like other steroids, impair the degradation of bilirubin, especially during the first week of life. If the mother has received NORETHISTERONE ENANTHATE INJECTION, breast-feeding should therefore be withheld from neonates with severe or persistent jaundice requiring medical treatment.
Paediatric use :
NORETHISTERONE ENANTHATE INJECTION are not indicated for children.
INTERACTIONS :
The effectiveness of norethisterone enanthate may be reduced when it is taken with :
- Rifamycins such as rifabutin and rifampicin.
- Antiepileptics such as carbamazepine, phenytoin phenobarbital, primidone and topiramate.
- Antiviral medicines such as nevirapine.
- Anti-bacterials such as tetracycline, chloramphenicol, metronidazole.
Barrier contraception must be used whilst taking any of these combinations, and should be continued for seven days after stopping the other medicine. Norethisterone enanthate may increase the blood levels of cyclosporin and individuals taking this combination should be carefully monitored. Norethisterone may affect the control of diabetes. Individuals taking anti-diabetic medicines together with this contraceptive should monitor their blood sugar levels and the dose of the anti diabetic adjusted if necessary.
SIDE EFFECTS :
Side-effects of progestogens include menstrual disturbances, premenstrual-like syndrome (including bloating, fluid retention, breast tenderness), weight change, nausea, headache, dizziness, insomnia, drowsiness, depression, change in libido; also skin reactions (including urticaria, pruritus, rash, and acne), hirsutism and alopecia. Jaundice and anaphylactoid reactions have also been reported. Parenteral preparations reliably inhibit ovulation and therefore protect against ectopic pregnancy and functional ovarian cysts.
OVERDOSAGE :
Presentation of a single use injectable and administration by a physician minimize the risk of overdose. There have been no reports of serious deleterious effects from overdose.
TREATMENT OF OVERDOSAGE :
There are no antidotes and further treatment should be symptomatic.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C, protected from light.
Do not refrigerate.
Warming and rotating the ampoule between the palms of the hands will redissolve any crystals that may have formed during storage at low temperatures.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
NORETHISTERONE ENANTHATE INJECTION is supplied as per below table :
| Strength | Pack Size | Pack |
| 100 mg/ml | 1 ml Ampoule | Single Ampoule |
| 200 mg/ml | 1 ml Ampoule | Single Ampoule |
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





