
50 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
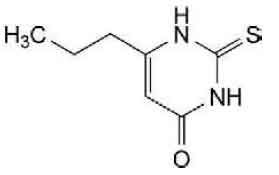
PROPYLTHIOURACIL TABLETS B.P. (Propylthiouracil) is a thiourea antithyroid drug. Chemically, Propylthiouracil is 2,3-dihydro-6-propyl-2-thioxopyrimidin-4(1H)-one. The molecular formula is C7H10N2OS and molecular weight is 170.2.
STRUCTURAL FORMULA :
Its structural formula is :
PROPYLTHIOURACIL TABLETS B.P. is white coloured, circular, biconvex tablet having breakline on one side and other side plain.
COMPOSITION :
Each uncoated tablet contains :
Propylthiouracil B.P. 50 mg
Excipients q.s.
ACTIONS :
Propylthiouracil inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triodothyronine that are stored in the thyroid or circulating in the blood, nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection. Propylthiouracil inhibits the conversation of thyroxine to triiodothyronine to peripheral tissues and may therefore be an effective treatment for thyroid storm.
PHARMACOKINETICS :
Propylthiouracil is rapidly absorbed from the gastrointestinal tract with a 50 to 75 % bioavailability and with peak plasma concentrations occurring about 2 hours after oral doses. It is concentrated in the thyroid gland; since its duration of action is more closely related to the intrathyroidal drug concentration than its plasma half-life, prolonged antithyroid activity results from single daily doses. Propylthiouracil is about 80 % bound to plasma proteins. Propylthiouracil has an elimination half-life of about 1 to 2 hours. It undergoes rapid first-pass metabolism in the liver, and is mainly excreted in the urine as the glucuronic acid conjugate, with less than 2 % excreted as unchanged drug. The elimination half-life may be increased in renal or hepatic impairment. Propylthiouracil crosses the placenta and is distributed into breast milk.
INDICATIONS :
PROPYLTHIOURACIL TABLETS B.P. is indicated in patients with hyperthyroidism (including Graves’ disease).
Administration :
PROPYLTHIOURACIL TABLETS B.P. is for oral administration.
Dosage :
Adults :
Propylthiouracil is given in a dose of 200 to 400 mg daily in adults and this dose is maintained until the patient becomes euthyroid; the dose may then be gradually reduced to a maintenance dose of 50 to 150 mg daily and continued for 1 to 2 years.
Children :
Neonate :
Initially 2.5 - 5 mg / kg twice daily until euthyroid then adjusted as necessary; higher doses occasionally required, particularly in thyrotoxic crisis.
Child 1 month – 1 year :
Initially 2.5 mg / kg 3 times daily until euthyroid then adjusted as necessary; higher doses occasionally required, particularly in thyrotoxic crisis.
Child 1 – 5 years :
Initially 25 mg 3 times daily until euthyroid then adjusted as necessary; higher doses occasionally required, particularly in thyrotoxic crisis.
Child 5 – 12 years :
Initially 50 mg 3 times daily until euthyroid then adjusted as necessary; higher doses occasionally required, particularly in thyrotoxic crisis.
Child 12 – 18 years :
Initially 100 mg 3 times daily administered until euthyroid then adjusted as necessary; higher doses occasionally required, particularly in thyrotoxic crisis.
CONTRAINDICATIONS :
PROPYLTHIOURACIL TABLETS B.P. is contraindicated in patients with known hypersensitivity to Propylthiouracil or to any of the excipients. PROPYLTHIOURACIL TABLETS B.P. is contraindicated in patients with previous severe hypersensitivity reaction e.g. agranulocytosis, hepatitis, vasculitis, nephritis. PROPYLTHIOURACIL TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
Liver Toxicity :
Liver injury resulting in liver failure, liver transplantation, or death, has been reported with Propylthiouracil therapy in adult and paediatric patients. No cases of liver failure have been reported with the use of methimazole in paediatric patients. For this reason, Propylthiouracil is not recommended for paediatric patients except when methimazole is not well-tolerated and surgery or radioactive iodine therapy are not appropriate therapies. There are cases of liver injury, including liver failure and death, in women treated with Propylthiouracil during pregnancy. Two reports of in utero exposure with liver failure and death of a newborn have been reported. The use of an alternative antithyroid medication (e.g., methimazole) may be advisable following the first trimester of pregnancy.
Biochemical monitoring of liver function (bilirubin, alkaline phosphatase) and hepatocellular integrity (ALT, AST) is not expected to attenuate the risk of severe liver injury due to its rapid and unpredictable onset. Patients should be informed of the risk of liver failure. Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc.) particularly in the first six months of therapy. When these symptoms occur, propylthouracil should be discontinued immediately and liver function tests and ALT and AST levels obtained.
Agranulocytosis :
Agranulocytosis occurs in approximately 0.2 % to 0.5 % of patients and is a potentially life-threatening side effect of Propylthiouracil therapy. Agranulocytosis typically occurs within the first 3 months of therapy. Patients should be instructed to immediately report any symptoms suggestive of agranulocytosis, such as fever or sore throat. Leucopenia, thrombocytopenia, and aplastic anaemia (pancytopenia) may also occur. Propylthiouracil should be discontinued if agranulocytosis, aplastic anaemia (pancytopenia), ANCA-positive vasculitis, hepatitis, interstitial pneumonitis, fever, or exfoliative dermatitis is suspected, and the patient’s bone marrow indices should be obtained.
Hypothyroidism :
Propylthiouracil can cause hypothyroidism necessitating routine monitoring of TSH and free T4 levels with adjustments in dosing to maintain a euthyroid state. Because the drug readily crosses placental membranes, Propylthiouracil can cause foetal goiter and cretinism when administered to a pregnant woman.
PRECAUTIONS :
General :
Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, jaundice, light colored stools, dark urine, right upper quadrant pain, etc.), particularly in the first six months of therapy. When these symptoms occur, measurement should be made of liver function (bilirubin, alkaline phosphatase) and hepatocellular integrity (ALT/AST levels). Patients who receive PROPYLTHIOURACIL TABLETS B.P. should be under close surveillance and should be counseled regarding the necessity of reporting any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. In such cases, white blood cell and differential counts should be obtained to determine whether agranulocytosis has developed. Particular care should be exercised with patients who are receiving concomitant drugs known to be associated with agranulocytosis. PROPYLTHIOURACIL TABLETS B.P. should be used cautiously in diabetic patients.
Pregnancy : Category D
Because propylthiouracil readily crosses placental membranes and can induce goiter and even cretinism in the developing foetus, it is important that a sufficient, but not excessive, dose be given during pregnancy. In many pregnant women, the thyroid dysfunction diminishes as the pregnancy proceeds; consequently a reduction of dosage may be possible. In some instances, propylthiouracil can be withdrawn several weeks or months before delivery.
Nursing mothers :
Propylthiouracil also transfers to breast milk but this does not preclude breast-feeding. Neonatal development and infant thyroid function should be closely monitored. The lowest effective dose should be used.
Paediatric Use :
Post-marketing reports of severe liver injury including hepatic failure requiring liver transplantation or resulting in death have been reported in the paediatric population. No such reports have been observed with methimazole. As such, Propylthiouracil is not recommended for use in the paediatric population except in rare instances in which methimazole is not well-tolerated and surgery or radioactive iodine therapy are not appropriate. When used in children, parents and patients should be informed of the risk of liver failure. If patients taking Propylthiouracil develop tiredness, nausea, anorexia, fever, pharyngitis, or malaise, Propylthiouracil should be discontinued immediately by the patient, a physician should be contacted, and a white blood cell count, liver function tests, and transaminase levels obtained.
Carcinogenicity / Mutagenicity :
Thyroid hyperplasia and carcinoma have occurred in laboratory animals treated with propylthiouracil for longer than 1 year. Similar effects are seen with continuous thyroid suppression with various antithyroid agents, dietary iodine deficiency, subtotal thyroidectomy, and ectopic thyrotropin-secreting pituitary tumors. Pituitary adenomas have also occurred.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
PROPYLTHIOURACIL TABLETS B.P. may cause drowsiness, which can hamper the patient’s ability to drive and use machinery.
INTERACTIONS AND INCOMPATIBILITIES :
Anticoagulants (Oral) :
Due to the potential inhibition of vitamin K activity by Propylthiouracil, the activity of oral anticoagulants (e.g., warfarin) may be increased; additional monitoring of PT/INR should be considered, especially before surgical procedures.
Beta-adrenergic blocking agents :
Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A reduced dose of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis Glycosides :
Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dosage of digitalis glycosides may be needed.
Theophylline :
Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
SIDE EFFECTS :
Blood and lymphatic system :
Reversible leucopenia. Rarely, agranulocytosis, thrombocytopenia, leucopenia, aplastic anaemia, pancytopenia. A rare complication of therapy is a tendency to haemorrhage associated with hypoprothrombinaemia which may be controlled by the administration of phytomenadione.
Ear and labyrinth disorders :
Rarely, hearing impairment may occur with propylthiouracil. The impairment usually becomes less marked after withdrawal of the drug.
Gastrointestinal :
Nausea, gastrointestinal disturbances, taste perversion. Rarely vomiting.
General :
Fever.
Hepatobiliary :
Jaundice (usually cholestatic), hepatic necrosis (sometimes with fatal consequences), encephalopathy. More commonly, asymptomatic liver function test abnormalities (increased serum bilirubin, Alanine transaminase and / or alkaline phosphatase concentrations), which are reversible on dose reduction or discontinuation of treatment, may occur with propylthiouracil.
Frequency unknown :
Hepatitis, hepatic failure.
Immune system :
Interstitial pneumonitis, alveolar haemorrhage, lymphadenopathy, arthritis, nephritis, vasculitis and lupus erythematosus-like syndromes have occurred in some patients taking thiourea antithyroid drugs. An immune mechanism has been proposed. There have also been rare reports of acute glomerulonephritis. Hypersensitivity reactions may also be associated with the development of antineutrophil cytoplasmic antibodies (ANCA).
Musculoskeletal :
Myopathy, arthralgia.
Nervous system :
Headache.
Skin :
Mild papular skin rashes, pruritus, urticaria, alopecia, cutaneous vasculitis.
INFORMATION FOR PATIENTS :
Patients should be advised that if they become pregnant or intend to become pregnant while taking an antithyroid drug, they should contact their physician immediately about their therapy. Patients should report immediately any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. They also should report symptoms suggestive of hepatic dysfunction (anorexia, pruritis, right upper quadrant pain, etc).
OVERDOSAGE :
Symptoms :
Overdose may manifest as Nausea, vomiting, epigastric distress, headache, fever, arthralgia, pruritus, edema, and pancytopenia. Agranulocytosis is the most serious effect, Rarely, exfoliative dermatitis, hepatitis, neuropathies, or CNS stimulation or depression may occur.
TREATMENT OF OVERDOSAGE :
The treatment of propylthiouracil overdose should aim to minimise the amount of drug absorbed into the circulation. Treatment should involve liberal use of oral fluids. Activated charcoal may also be employed. General symptomatic and supportive measures should then be instituted. A full blood analysis should be considered because of the slight risk of haematological complications and appropriate therapy given if bone marrow depression develops. There is no specific antidote for propylthiouracil.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
PROPYLTHIOURACIL TABLETS B.P. contains Propylthiouracil B.P. 50 mg.
3 Strips of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





