
250 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
CHLORAMPHENICOL CAPSULES B.P. (Chloramphenicol) is a broad-spectrum antibiotic effective against a wide range of Gram-negative & Gram-positive bacteria, rickettsias, and chlamydias. Chemically, Chloramphenicol is 2,2-dichloro-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide. The molecular formula is C11H12Cl2N2O5 and molecular weight is 323.1.
STRUCTURAL FORMULA :
Its structural formula is :
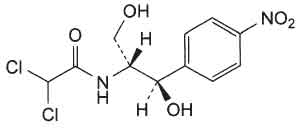
CHLORAMPHENICOL CAPSULES B.P. contains yellowish white powder filled in hard gelatin capsule of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Chloramphenicol B.P. 250 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Chloramphenicol is a bacteriostatic antibiotic with a broad spectrum of action against both Gram-positive and Gram-negative bacteria, as well as some other organisms.
Mechanism of action :
Chloramphenicol is thought to enter sensitive cells by an active transport process. Within the cell it binds to the 50S subunit of the bacterial ribosome at a site adjacent to the site of action of the macrolides and clindamycin, and inhibits bacterial protein synthesis by preventing attachment of aminoacyl transfer RNA to its acceptor site on the ribosome, thus preventing peptide bond formation by peptidyl transferase. The block in protein synthesis results in a primarily bacteriostatic action, although it may be bactericidal to some organisms, including Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae, at higher concentrations.
Spectrum of activity :
Chloramphenicol has activity against many types of bacteria, although in most cases there are less toxic alternatives available. The following pathogens are usually susceptible. Gram-positive cocci including staphylococci such as Staph. epidermidis and some strains of Staph. aureus, and streptococci such as Str., pneumoniae, Str. pyogenes, and the viridans streptococci. Meticillin-resistant staphylococci and Enterococcus faecalis are commonly found to be resistant. Other Gram-positive species including Bacillus anthracis, Corynebacterium diphtheriae, and anaerobes such as Peptococcus and Peptostreptococcus spp. are usually susceptible.Gram-negative cocci such as Neisseria meningitidis and N. gonorrhoeae are usually highly sensitive, as are Haemophilus influenzae and a variety of other Gram-negative bacteria including Bordetella pertussis, Brucella abortus, Campylobacter spp., Legionella pneumophila, Pasteurella, and Vibrio spp. The Enterobacteriaceae vary in their susceptibility, and many strains have shown acquired resistance, but Escherichia coli, and strains of Klebsiella spp., Proteus mirabilis, Salmonella, Shigella, and Yersinia spp. have been reported to be susceptible. Many strains of Enterobacter, indole-positive Proteus, and Serratia spp. are resistant, or at best moderately susceptible. Pseudomonas aeruginosa is invariably resistant, although Burkholderia (formerly Pseudomonas) spp. may be susceptible. Some Gram-negative anaerobes are susceptible, or moderately so, including Bacteroides fragilis, Veillonella, and Fusobacterium spp. Other susceptible organisms include Actinomyces spp., Leptospira spp., spirochaetes such as Treponema pallidum, Chlamydiaceae, Mycoplasma spp., and Rickettsia spp. Nocardia spp. are resistant. Chloramphenicol is ineffective against fungi, protozoa, and viruses.
Activity with other antimicrobials :
As with other bacteriostatic antimicrobials, the possibility exists of an antagonistic effect if chloramphenicol is given with a bactericidal drug, and some antagonism has been demonstrated in vitro between chloramphenicol and various beta lactams and aminoglycosides, but the clinical significance of most of these interactions is usually held to be doubtful. Chloramphenicol may competitively inhibit the effects of macrolides or lincosamides such as clindamycin because of the adjacency of their binding sites on the ribosome.
Resistance :
Acquired resistance has been widely reported, although the prevalence of resistance has tended to decline where use of the drug has become less frequent. The most commonly seen form of resistance has been the production of an acetyltransferase that inactivates the drug. Such resistance is usually plasmidmediated and may be associated with resistance to other drugs such as the tetracyclines. Other mechanisms that may reduce sensitivity to chloramphenicol include reduced permeability or uptake, and ribosomal mutation. The actual incidence of resistance varies considerably in different countries and different centres. Epidemics of chloramphenicol-resistant Salmonella and Shigella spp. have occurred in the past, and although the prevalence of resistance in Salmonella spp. has been reported to be negligible except in parts of South or Southeast Asia, resistant salmonellal infections acquired in these regions are increasingly being seen elsewhere. Resistance among Haemophilus and Neisseria spp. occurs, and the latter may be problematic in developing countries, although it does not yet seem to be widespread. However, resistant strains of enterococci and pneumococci are reported to be relatively common in some areas, and over 50 % of staphylococcal strains have been reported to show resistance in some hospitals.
PHARMACOKINETICS :
Chloramphenicol is readily absorbed when given by mouth. Blood concentrations of 10 micrograms/ml or more may be reached about 1 or 2 hours after a single oral dose of 1g, and blood concentrations of about 18.5 micrograms/ml have been reported after multiple 1-g doses. Chloramphenicol is widely distributed in body tissues and fluids; it enters the CSF, giving concentrations of about 50 % of those existing in the blood even in the absence of inflamed meninges; it diffuses across the placenta into the foetal circulation, into breast milk, and into the aqueous and vitreous humours of the eye. It also enters the aqueous humour after topical application. Up to about 60 % in the circulation is bound to plasma protein. The half-life of chloramphenicol has been reported to range from 1.5 to 4 hours; the
half-life is prolonged in patients with severe hepatic impairment and is also much longer in neonates. Renal impairment has relatively little effect on the half-life of the active drug, due to its extensive metabolism, but may lead to accumulation of the inactive metabolites.
Chloramphenicol is excreted mainly in the urine but only 5 to 10 % of an oral dose appears unchanged; the remainder is inactivated in the liver, mostly by conjugation with glucuronic acid. About 3 % is excreted in the bile. However, most is reabsorbed and only about 1 %, mainly in the inactive form, is excreted in the faeces. The absorption, metabolism, and excretion of chloramphenicol are subject to considerable interindividual variation, especially in infants and children, making monitoring of plasma concentrations necessary to determine pharmacokinetics in a given patient.
INDICATIONS :
Brain abscess (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of brain abscess caused by B. fragilis or other susceptible organisms.
Ehrlichiosis (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of ehrlichiosis caused by Ehrlichia canis.
Meningitis (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of meningitis caused by H. influenzae, S. pneumoniae, and N. meningitidis.
Paratyphoid fever (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of paratyphoid fever caused by S. paratyphi A.
Q fever (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of Q fever caused by Coxiella burnetii.
Rocky Mountain spotted fever (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of Rocky Mountain spotted fever caused by Rickettsia species.
Typhoid fever (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the acute treatment of typhoid fever only, caused by S. typhi.
Typhus infections (treatment) : CHLORAMPHENICOL CAPSULES B.P. is indicated in the treatment of typhus infections caused by Rickettsia species.
Not all species or strains of a particular organism may be susceptible to chloramphenicol.
Administration :
CHLORAMPHENICOL CAPSULES B.P. are for oral use.
CHLORAMPHENICOL CAPSULES B.P. should be swallowed whole.
CHLORAMPHENICOL CAPSULES B.P. should preferably be taken with a full glass (240 ml) of water on an empty stomach (either 1 hour before or 2 hours after meals) to optimize absorption.
Dosage :
50 mg/kg daily in 4 divided doses (exceptionally, can be doubled for severe infections such as septicaemia and meningitis, providing high doses reduced as soon as clinically indicated).
CHILD :
Haemophilus epiglottitis and pyogenic meningitis, 50–100 mg/kg daily in divided doses (high dosages decreased as soon as clinically indicated).
NEONATE :
Under 2 weeks 25 mg/kg daily (in 4 divided doses).
INFANT :
2 weeks – 1 year 50 mg/kg daily (in 4 divided doses).
CONTRAINDICATIONS :
CHLORAMPHENICOL CAPSULES B.P. is contra-indicated in the following conditions :
1.Patients with a history of hypersensitivity or allergy or toxic reactions.
2.For minor infections or for prophylaxis.
3.Aplastic anaemia.
4.During active immunisation.
WARNINGS :
The risk of aplastic anaemia does not restrict the use of chloramphenicol in situations in which it is necessary; however, it emphasizes that the drug should never be employed in diseases readily, safely and effectively treatable with other antimicrobial agents or in undefined cases. Because of the risk of the “grey syndrome”, neonates should only be given chloramphenicol when it may be life-saving and no alternative treatment is available. Repeated courses and prolonged treatment should be avoided. Concomitant administration of chloramphenicol with other drugs liable to depress bone-marrow function should be avoided. Chloramphenicol enhances the effects of coumarin anticoagulants, some hypoglycaemic agents (chlorpropamide and tolbutamide) and phenytoin.
PRECAUTIONS :
Hypersensitivity reactions :
Macular or vesicular skin rashes occur as a result of hypersensitization to chloramphenicol. Fever may appear simultaneously or be the sole manifestation. Angioedema is a rare complication. Herxheimer reactions have been observed. Erythema multiforme has been reported.
Haematological Toxicity :
The most important adverse effect of chloramphenicol is on the bone-marrow. Changes in peripheral blood include leucopenia, thrombocytopaenia, and aplasia of the marrow with fatal pancytopenia. The incidence is not dose-related. The fatality rate is high when bone-marrow aplasia is complete, and there is a high incidence of acute leukaemia in those who recover. A second haematological effect of chloramphenicol is a predictable but reversible erythroid suppression of the bone marrow. The clinical picture is featured initially by reticulopenia, which occurs 5 to 7 days after the initiation of therapy, followed by a decrease in haemoglobin, an increase in plasma iron, cytoplasmic vacuolation of early erythroid forms and granulocyte precursors, and normoblastosis with a shift to early erythrocyte forms. Severe leucopenia and thrombocytopaenia may also occur. The incidence and severity of this syndrome are dose-related. It occurs regularly when plasma concentrations are 25µg/ml or higher and is observed during the use of large doses of chloramphenicol, prolonged treatment, or both. Haemolytic anaemia has occurred in persons with a genetic deficiency of glucose-6-phosphate dehydrogenase activity.
Other Effects:
Gastrointestinal symptoms including nausea, vomiting, unpleasant taste, diarrhoea and perineal irritation may follow oral administration of chloramphenicol. Disturbances of the oral and intestinal flora may cause stomatitis, glossitis, and rectal or vaginal irritation. Prolonged oral administration of chloramphenicol may induce bleeding either by bone-marrow depression or by reducing the intestinal flora with consequent inhibition of vitamin K synthesis and greatly increased prothrombin time. Among the toxic effects produced by chloramphenicol are blurring of vision and digital paresthesias. Peripheral as well as optic neuritis has been reported in patients receiving chloramphenicol, usually over prolonged periods. A toxic manifestation the “grey syndrome” has occurred in premature and other newborn infants receiving large doses of chloramphenicol. A similar syndrome has been reported in adults and children given high doses. Chloramphenicol should never be given for minor infections or for prophylaxis and repeated courses and prolonged treatment should be avoided.
Reduced doses should be given to patients with impaired liver function. Uraemic patients may be more susceptible to the depressant effect of chloramphenicol on bone-marrow but reducing the dose may result in inadequate plasma concentrations. Routine periodic blood examinations are advisable in all patients including infants; these examinations will not warn of aplastic anaemia. Because of the risk of the “grey syndrome” newborn infants should not be given chloramphenicol, unless it may be lifesaving and there is no alternative treatment. The use of chloramphenicol is best avoided during pregnancy; nursing infants should be observed with care since chloramphenicol given to the mother is excreted in the milk.
Pregnancy : Category C
Chloramphenicol readily crosses the placenta; foetal serum concentrations may be 30 to 80 % of maternal serum concentrations. Although birth defects in humans have not been documented, use is not recommended in pregnancy at term or during labour because of potential toxicity (“gray syndrome” or bone marrow depression) in premature or full-term infants.
Nursing mothers :
Chloramphenicol is excreted in breast milk in concentrations up to 25 mcg per ml. Use is not recommended in nursing mothers because of the possibility of adverse effects, especially bone marrow depression, in the infant.
Paediatric Use :
In the foetus and neonates, the immature liver cannot conjugate chloramphenicol, and toxic concentrations of active drug accumulate. In neonates and infants this may result in the “gray syndrome”. In infants 1 to 2 days old, the half-life is 24 hours or longer and may be highly variable, especially in low-birth-weight infants. In infants 10 to 16 days old, the half-life is 10 hours. Inactive metabolites can accumulate in premature and newborn infants because of immaturity of renal tubular secretion mechanisms. Serum concentrations must be performed in paediatric patients with impaired or immature metabolic functions or in patients who are receiving medications that are also metabolized by the liver (e.g., phenytoin, phenobarbital, acetaminophen, theophylline).
INTERACTIONS AND INCOMPATIBILITIES :
Anticoagulants :
May enhance anticoagulation action.
Barbiturates :
May reduce effectiveness of chloramphenicol while barbiturate effects may be enhanced; effects may last days after barbiturates are withdrawn.
Ferrous salts :
May increase serum iron levels.
Hydantoins (eg, phenytoin) :
May increase serum hydantoin levels, with possible toxicity; chloramphenicol levels may increase or decrease.
Rifampin :
May reduce chloramphenicol serum levels; effect may last days after rifampin is withdrawn.
Sulfonylureas :
May cause clinical manifestations of hypoglycaemia.
Vitamin B12 :
May decrease haematologic effects of vitamin B12 in patients with pernicious anaemia.
SIDE EFFECTS :
Blood disorders including reversible and irreversible aplastic anaemia (with reports of resulting leukaemia), peripheral neuritis, optic neuritis, headache, depression, urticaria, erythema multiforme, nausea, vomiting, diarrhoea, stomatitis, glossitis, dry mouth; nocturnal haemoglobinuria reported; “grey syndrome” (abdominal distension, pallid cyanosis, circulatory collapse) may follow excessive doses in neonates with immature hepatic metabolism.
OVERDOSAGE :
The “grey syndrome” : characterised by vomiting, abdominal distension, ashen colour, hypothermia, irregular respiration, progressive pallid cyanosis, and shock, followed by death in a few hours or days. Other toxic manifestations include aplastic anaemia, blurring of vision, digital paraesthesias, optic neuritis, allergic skin rashes, and gastro-intestinal haemorrhage.
TREATMENT OF OVERDOSAGE :
Chloramphenicol should be discontinued immediately on the appearance of toxic symptoms. Treatment is symptomatic and supportive.
STORAGE :
Store below 30°C (86°F), protect from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CHLORAMPHENICOL CAPSULES B.P. contains Chloramphenicol B.P. 250 mg.
3 Blisters of 10 Capsules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





