1 gm
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
SUCRALFATE TABLETS USP (Sucralfate) is an (alpha)-D-glucopyranoside, (Beta)-D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. Chemically, sucralfate is -D-Glucopyranoside, -D-fructofuranosyl-, octakis(hydrogen sulfate), aluminum complex.
The molecular formula is C12H54 Al16O75S8 and molecular weight is 2086.72.
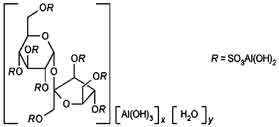
STRUCTURAL FORMULA :
Its structural formula is :
SUCRALFATE TABLETS USP is white coloured capsule or oval shaped uncoated tablet.
COMPOSITION :
Each uncoated tablet contains :
Sucralfate USP 1 gm
Excipients q.s.
ACTIONS :
Exact mechanism of action is not known; however, sucralfate is thought to form an ulcer-adherent complex with proteinaceous exudate, such as albumin and fibrinogen, at the ulcer site, protecting it against further acid attack. To a lesser extent, sucralfate forms a viscous, adhesive barrier on the surface of intact mucosa of the stomach and duodenum. Sucralfate also inhibits pepsin activity and has been found to bind bile salts in vitro. Recent information suggests that sucralfate may increase the production of prostaglandin E2 and gastric mucous.
PHARMACOKINETICS :
Absorption :
Up to 5 % of the disaccharide component and less than 0.02 % of aluminum is absorbed from the gastrointestinal tract following an oral sucralfate dose.
Elimination :
Mostly faecal; small amounts of sulfate disaccharide are eliminated in the urine.
INDICATIONS :
SUCRALFATE TABLETS USP is indicated in :
• Short-term treatment (up to 8 weeks) of active duodenal ulcer. While healing with sucralfate may occur during the first week or two, treatment should be continued for 4 to 8 weeks unless healing has been demonstrated by x-ray or endoscopic examination.
• Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers.
Administration :
For oral use.
Dosage :
Active Duodenal Ulcer : The recommended adult oral dosage for duodenal ulcer is 1 g four times per day on an empty stomach. Antacids may be prescribed as needed for relief of pain but should not be taken within one-half hour before or after SUCRALFATE TABLETS USP. While healing with SUCRALFATE TABLETS USP may occur during the first week or two, treatment should be continued for 4 to 8 weeks unless healing has been demonstrated by x-ray or endoscopic examination.
Maintenance Therapy :
The recommended adult oral dosage is 1 g twice a day.
CONTRAINDICATIONS :
There are no known contraindications to the use of SUCRALFATE TABLETS USP.
WARNINGS :
Proper diagnosis is important since symptomatic response to sucralfate therapy does not preclude the presence of a gastric malignancy. There is no clinical experience in the use of sucralfate in patients with actively haemorrhaging ulcers. Recurrence may be observed in patients with gastric or duodenal ulcers. While the treatment with sucralfate can result in complete healing of ulcer, a successful course of treatment should not be expected to alter the underlying cause of ulcer disease. The risk of recurrence of duodenal ulcers may be reduced by maintaining the patients on a reduced dose for up to 12 months after healing is complete; see Dosage and Administration.
PRECAUTIONS :
Duodenal ulcer is a chronic, recurrent disease. While short-term treatment with sucralfate can result in complete healing of the ulcer, a successful course of treatment with sucralfate should not be expected to alter the posthealing frequency or severity of duodenal ulceration.
Special Populations :
Chronic Renal Failure and Dialysis Patients :
When sucralfate is administered orally, small amounts of aluminum are absorbed from the gastrointestinal tract. Concomitant use of sucralfate with other products that contain aluminum, such as aluminum-containing antacids, may increase the total body burden of aluminum. Patients with normal renal function receiving the recommended doses of sucralfate and aluminum-containing products adequately excrete aluminum in the urine. Patients with chronic renal failure or those receiving dialysis have impaired excretion of absorbed aluminum. In addition, aluminum does not cross dialysis membranes because it is bound to albumin and transferrin plasma proteins. SUCRALFATE TABLETS USP should be used with caution in patients with chronic renal failure.
Pregnancy :
Teratogenic effects. Pregnancy Category B
Teratogenicity studies have been performed in mice, rats, and rabbits at doses up to 50 times the human dose and have revealed no evidence of harm to the foetus due to sucralfate. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when sucralfate is administered to a nursing woman.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Some studies have shown that simultaneous sucralfate administration in healthy volunteers reduced the extent of absorption (bioavailability) of single doses of the following cimetidine, digoxin, fluoroquinolone antibiotics, ketoconazole, I-thyroxine, phenytoin, quinidine, ranitidine, tetracycline, and theophylline. Subtherapeutic prothrombin times with concomitant warfarin and sucralfate therapy have been reported in spontaneous and published case reports. However, two clinical studies have demonstrated no change in either serum warfarin concentration or prothrombin time with the addition of sucralfate to chronic warfarin therapy.
The mechanism of these interactions appears to be nonsystemic in nature, presumably resulting from sucralfate binding to the concomitant agent in the gastrointestinal tract. In all cases studies to date (cimetidine, ciprofloxacin, digoxin, norfloxacin, ofloxacin, and ranitidine), dosing the concomitant medication 2 hours before sucralfate eliminated the interaction. Because of the potential of SUCRALFATE TABLETS USP to alter the absorption of some drugs, SUCRALFATE TABLETS USP should be administered separately from other drugs when alterations in bioavailabity are felt to be critical. In these cases, patients should be monitored appropriately.
SIDE EFFECTS :
Constipation has been encountered in about 2 to 3 % of patients in various trials. Other adverse effects include headache (2.4 %), Urticaria (1 %), nausea, diarrhea, gastric discomfort, indigestion, dry mouth, skin rash, pruritus, back pain, dizziness, sleepiness and vertigo. No additional side effects have been associated with maintenance use of sucralfate for up to 12 months at the recommended dose.
OVERDOSAGE :
Due to limited experience in humans with overdosage of sucralfate, no specific treatment recommendations can be given. Acute oral toxicity studies in animals, however, using doses up to 12 g/kg body weight, could not find a lethal dose. Sucralfate is only minimally absorbed from the gastrointestinal tract. Risks associated with acute overdosage should, therefore, be minimal. In rare reports describing sucralfate overdose, most patients remained asymptomatic. Those few reports where adverse events were described included symptoms of dyspepsia, abdominal pain, nausea, and vomiting.
STORAGE :
Store below 250C (770F), protected from light and humidity.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
SUCRALFATE TABLETS USP contains Sucralfate USP 1 gm.
One bottle of 100 tablets per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular