0.4 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
NALOXONE HYDROCHLORIDE INJECTION USP (Naloxone hydrochloride) is opioid antagonist. Chemically, naloxone hydrochloride is morphinan-6-one, 4,5-epoxy-3, 14-dihydroxy-17-(2-propenyl)-, hydrochloride, (5α)-. The molecular formula is C19H21NO4·HCl and molecular weight is 363.84.
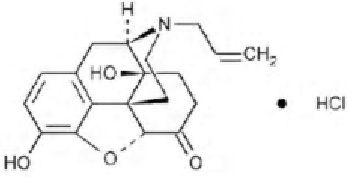
STRUCTURAL FORMULA :
Its structural formula is :
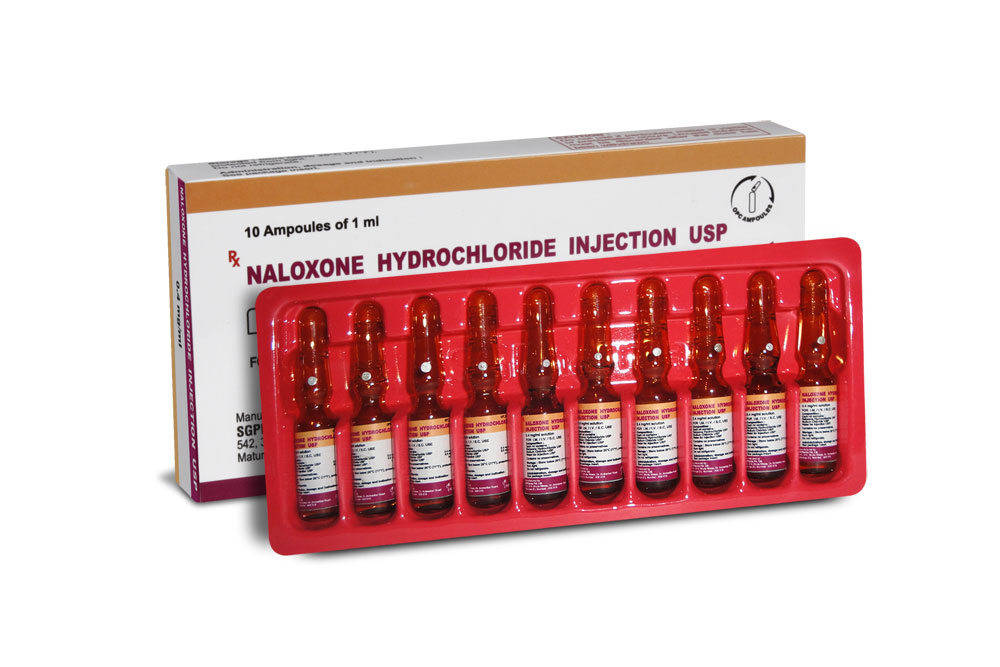
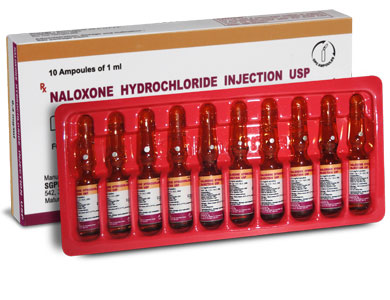
NALOXONE HYDROCHLORIDE INJECTION USP is a clear, colourless solution filled in 1 ml amber glass ampoule.
COMPOSITION :
Each ml contains :
Naloxone Hydrochloride USP 0.4 mg
Sodium Chloride USP 8.6 mg
Water for Injection USP q.s.
Contains no preservatives
ACTIONS :
Naloxone hydrochloride, a narcotic antagonist, is a synthetic congener of oxymorphone. Naloxone hydrochloride prevents or reverses the effects of opioids including respiratory depression, sedation and hypotension. Also, it can reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine. Naloxone hydrochloride is an essentially pure narcotic antagonist, i.e. it does not possess the “agonistic” or morphine-like properties characteristic of other narcotic antagonists. Naloxone hydrochloride does not produce respiratory depression, psychotomimetic effects of pupillary constriction. In the absence of narcotics or agonistic effects of other narcotic antagonists it exhibits essentially no pharmacologic activity. Naloxone hydrochloride has not been shown to produce tolerance nor to cause physical or psychological dependence. In the presence of physical dependence on narcotics naloxone hydrochloride will produce withdrawal symptoms. While the mechanism of action of naloxone hydrochloride is not fully understood, the preponderance of evidence suggests that naloxone hydrochloride antagonises the opioid effects by competing for the same receptor sites.
PHARMACOKINETICS :
Biotransformation :
Hepatic.
Half-life :
64 (range, 30 to 81) minutes.
Onset of action :
Intravenous : 1 or 2 minutes.
Intramuscular : 2 to 5 minutes.
Time to peak effect :
5 to 15 minutes.
Duration of action :
Dose and route dependent. In one study, effects persisted for 45 minutes following a 400 mcg (0.4 mg) intravenous dose. Intramuscular administration results in a prolonged duration of action.
Elimination :
Renal; about 70 % of a dose is excreted within 72 hours.
INDICATIONS :
NALOXONE HYDROCHLORIDE INJECTION USP is indicated for the complete or partial reversal of narcotic depression, including respiratory depression, induced by opioids including natural and synthetic narcotics, propoxyphene, methadone and the narcotic antagonist analgesics : nalbuphine, pentazocine and butorphanol. Naloxone hydrochloride is also indicated for the diagnosis of suspected acute opioid overdosage.
Administration :
NALOXONE HYDROCHLORIDE INJECTION USP may be administered intravenously, intramuscularly or subcutaneously. The most rapid onset of action is achieved by intravenous administration and it is recommended in emergency situations. Since the duration of action of some opioids may exceed that of naloxone the patient should be kept under continued surveillance. Repeated doses of naloxone should be administered, as necessary.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
By intravenous injection, 0.4–2 mg repeated at intervals of 2–3 minutes to a max. of 10 mg if respiratory function does not improve (then question diagnosis); child
10 micrograms/kg; if no response give subsequent dose of 100 micrograms/kg (then question diagnosis), further dose may be required if respiratory function deteriorates
By subcutneous or intramuscular injection, adult and child dose as for intravenous injection but use only if intravenous route not feasible (onset of action slower)
By continuous intravenous infusion using an infusion pump, 4 mg diluted in 20 ml intravenous infusion solution at a rate adjusted according to response (initial rate may be set at 60 % of initial intravenous injection dose (see above) and infused over 1 hour)
Important : Doses used in acute opioid overdosage may not be appropriate for the management of opioid-induced respiratory depression and sedation in those receiving palliative care and in chronic opioid use. Respiratory depression is a major concern with opioid analgesics and it may be treated by artificial ventilation or be reversed by NALOXONE HYDROCHLORIDE INJECTION USP. NALOXONE HYDROCHLORIDE INJECTION USP will immediately reverse opioid-induced respiratory depression but the dose may have to be repeated because of the short duration of action of naloxone; however, naloxone will also antagonise the analgesic effect.
CONTRAINDICATIONS :
NALOXONE HYDROCHLORIDE INJECTION USP is contraindicated in patients known to be hypersensitive to naloxone hydrochloride or any of the other ingredients contained in the formulation.
WARNINGS :
Drug Dependence :
NALOXONE HYDROCHLORIDE INJECTION USP should be administered cautiously to persons including newborns of mothers who are known or suspected to be physically dependent on opioids. In such cases, an abrupt and complete reversal of opioid effects may precipitate an acute withdrawal syndrome. The signs and symptoms of opioid withdrawal in a patient physically dependent on opioids may include, but are not limited to, the following : body aches, diarrhoea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness and increased blood pressure. In the neonate, opioid withdrawal may also include : convulsions, excessive crying, and hyperactive reflexes.
Repeat Administration :
The patient who has satisfactorily responded to naloxone should be kept under continued surveillance and repeated doses of naloxone should be administered, as necessary, since the duration of action of some opioids may exceed that of naloxone.
Respiratory Depression due to Other Drugs :
Naloxone is not effective against respiratory depression due to non-opioid drugs and in the management of acute toxicity caused by levopropoxyphene. Reversal of respiratory depression by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete or require higher doses of naloxone. If an incomplete response occurs, respirations should be mechanically assisted as clinically indicated.
Pregnancy : Category B
Teratogenic Effects :
Teratology studies conducted in mice and rats at doses 4-times and 8-times, respectively, the dose of a 50 kg human given 10 mg/day (when based on surface area or mg/m2), demonstrated no embryotoxic or teratogenic effects due to naloxone. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, naloxone hydrochloride should be used during pregnancy only if clearly needed.
Non-teratogenic effects :
Risk-benefit must be considered before naloxone is administered to a pregnant woman who is known or suspected to be opioid-dependent since maternal dependence may often be accompanied by foetal dependence. Naloxone crosses the placenta, and may precipitate withdrawal in the foetus as well as in the mother. Patients with mild to moderate hypertension who receive naloxone during labor should be carefully monitored as severe hypertension may occur.
Use in Labor and Delivery :
It is not known if NALOXONE HYDROCHLORIDE INJECTION USP affects the duration of labor and/or delivery. However, published reports indicated that the administration of naloxone during labor did not adversely affect maternal or neonatal status.
Nursing mothers :
It is not known whether naloxone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when naloxone hydrochloride is administered to a nursing woman.
Paediatric Use :
NALOXONE HYDROCHLORIDE INJECTION USP may be administered intravenously, intramuscularly or subcutaneously in children and neonates to reverse the effects of opiates. The American Academy of Paediatrics, however, does not endorse subcutaneous or intramuscular administration in opiate intoxication since absorption may be erratic or delayed. Although the opiate-intoxicated child responds dramatically to NALOXONE HYDROCHLORIDE INJECTION USP, he/she must be carefully monitored for at least 24 hours as a relapse may occur as naloxone is metabolized. When NALOXONE HYDROCHLORIDE INJECTION USP is given to the mother shortly before delivery, the duration of its effects lasts only for the first two hours of neonatal life. It is preferable to administer NALOXONE HYDROCHLORIDE INJECTION USP directly to the neonate if needed after delivery. Naloxone has no apparent benefit as an additional method of resuscitation in the newly born infant with intrauterine asphyxia, which is not related to opioid use.
Usage in Paediatric Patients and Neonates for
Septic Shock :
The safety and effectiveness of NALOXONE HYDROCHLORIDE INJECTION USP in the treatment of hypotension in paediatric patients and neonates with septic shock have not been established. One study of two neonates in septic shock reported a positive pressor response; however, one patient subsequently died after intractable seizures.
Geriatric Use :
Clinical studies of NALOXONE HYDROCHLORIDE INJECTION USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
Effects on ability to drive and operate machine :
NALOXONE HYDROCHLORIDE INJECTION USP may be likely to produce minor or moderate adverse effects that may impair the patient’s ability to concentrate and react and therefore constitute a risk in the ability to drive and use machines.
INTERACTIONS AND INCOMPATIBILITIES :
Naloxone reverses the analgesic and other effects of opioid agonist-antagonists such as pentazocine, so may precipitate withdrawal symptoms if used concurrently with these drugs in physically dependent patients. Naloxone reverses the analgesic and other effects of opioid agonist analgesics and may precipitate withdrawal symptoms if used concurrently with these drugs in physically dependent patients, including patients receiving methadone to treat opioid dependence. When naloxone is used post-operatively to reverse the central depressive effects of opioid agonists used as anaesthesia adjuncts, the dose of naloxone must be carefully titrated to achieve the desired effect without interfering with control of post-operative pain or causing other adverse effects.
SIDE EFFECTS :
Abrupt reversal of narcotic depression has been reported to result in nausea, vomiting, sweating, tachycardia, tremor and hyperventilation. In postoperative patients excessive dosage of naloxone may result in excitement, increased blood pressure and significant reversal of analgesia. Hypertension, pulmonary oedema, atrial and ventricular arrhythmias and cardiac arrest have been reported in certain patients, particularly those with pre-existing cardiac abnormalities. Seizures have occurred rarely following administration of naloxone, however, a casual relationship has not been established.
OVERDOSAGE :
There is no clinical experience with naloxone hydrochloride overdosage in humans. In mouse and rat, the intravenous LD50 is 150 ± 5 mg/kg and 109 ± 4 mg/kg respectively. In acute subcutaneous toxicity studies in newborn rats the LD50 (95 % CL) is 260 (228-296) mg/kg. Subcutaneous injection of 100 mg/kg/day in rats for 3 weeks produced only transient salivation and partial ptosis following injection, no toxic effects were seen at 10 mg/kg/day for 3 weeks.
Symptoms :
Symptoms of overdosage would be expected to be similar to the effects seen with therapeutic use.
TREATMENT OF OVERDOSAGE :
Treatment of overdosage is symptomatic and supportive.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
o not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
RESENTATION :
NALOXONE HYDROCHLORIDE INJECTION USP is supplied as 0.4 mg Naloxone hydrochloride USP in 1 ml aqueous solution.
Such 10 ampoules of 1 ml are packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular