100 mcg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
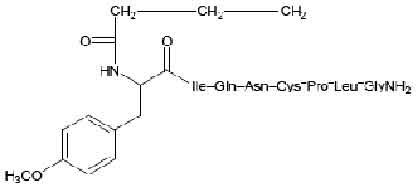
CARBETOCIN INJECTION is a oxytocic; antihaemorrhagic (postpartum uterine bleeding); uterine stimulant. Chemically, Carbetocin is 1-Butanoic acid-2-(O-methyl-L-tyrosine)-1-carbaoxytocin. The molecular formula is C45H69N11O12S and molecular weight is 988.18.
STRUCTURAL FORMULA :
Its structural formula is :
CARBETOCIN INJECTION is a sterile, clear, colourless solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Carbetocin 100 mcg
Sodium Chloride B.P. 9 mg
Water for Injections B.P. q.s.
Contains no preservatives.
ACTIONS :
The pharmacological and clinical properties of carbetocin are those of a long acting oxytocin agonist. Like oxytocin, carbetocin selectively binds to oxytocin receptors in the smooth muscle of the uterus, stimulates rhythmic contractions of the uterus, increases the frequency of existing contractions, and raises the tone of the uterus musculature. On the postpartum uterus, carbetocin is capable of increasing the rate and force of spontaneous uterine contractions. The onset of uterine contraction following carbetocin is rapid, with a firm contraction being obtained within 2 minutes. A single 100 micrograms intravenous dose of carbetocin administered after the delivery of the infant is sufficient to maintain adequate uterine contraction that prevents uterine atony and excessive bleeding comparable with an oxytocin infusion lasting for several hours.
PHARMACOKINETICS :
After intravenous injection of carbetocin, firm uterine contraction occurs within 2 minutes and lasts for several hours. Carbetocin undergoes a biphasic elimination, with a terminal elimination half-life of about 40 minutes. Less than 1 % of a dose is excreted unchanged by the kidney. Carbetocin is distributed into breast milk.
INDICATIONS :
CARBETOCIN INJECTION is indicated for the prevention of uterine atony and excessive bleeding following delivery of the infant by Caesarean section under epidural or spinal anaesthesia.
Administration :
For I.V. use only.
Administer 1 ml of CARBETOCIN INJECTION containing 100 micrograms carbetocin by intravenous injection, under adequate medical supervision in a hospital.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
A single dose of CARBETOCIN INJECTION must be administered slowly, over 1 minute, only after delivery of the infant by Caesarean section. It should be given as soon as possible after delivery, preferably before removal of the placenta. No further doses of CARBETOCIN INJECTION should be administered.
CONTRAINDICATIONS :
• During pregnancy and labour before delivery of the infant.
• CARBETOCIN INJECTION must not be used for the induction of labour.
• Hypersensitivity to carbetocin, oxytocin or to any of the excipients.
• Hepatic or renal disease.
• Cases of pre-eclampsia and eclampsia.
• Serious cardiovascular disorders.
• Epilepsy.
WARNINGS AND PRECAUTIONS :
CARBETOCIN INJECTION is intended for use only at well equipped specialist obstetrics units with experienced and qualified staff available at all times. The use of carbetocin at any stage before delivery of the infant is not appropriate because its uterotonic activity persists for several hours after a single bolus injection. This is in marked contrast to the rapid reduction of effect observed after discontinuation of an oxytocin infusion. In case of persistent uterine bleeding after administration of carbetocin the cause must be determined. Consideration should be given to causes such as retained placental fragments, inadequate emptying or repair of the uterus, or disorders of blood coagulation. CARBETOCIN INJECTION is intended for single administration only. It must be administered slowly over 1 minute. In case of persisting uterine hypotonia or atonia and the consequent excessive bleeding, additional therapy with oxytocin and/or ergometrine should be considered. There are no data on additional doses of carbetocin or on the use of carbetocin following persisting uterine atony after oxytocin.
Animal studies have shown carbetocin to possess some antidiuretic activity (vasopressin activity : < 0,025 IU/ampoule) and therefore the possibility of hyponatraemia cannot be excluded, particularly in patients also receiving large volumes of intravenous fluids. The early signs of drowsiness, listlessness and headache should be recognised to prevent convulsions and coma. In general, CARBETOCIN INJECTION should be used cautiously in the presence of migraine, asthma and cardiovascular disease or any state in which a rapid
addition to extracellular water may produce hazard for an already overburdened system. The decision of administering carbetocin can be made by the physician after carefully weighing the potential benefit carbetocin may provide in these particular cases. Specific studies have not been undertaken in gestational diabetes mellitus.The efficacy of carbetocin has not been assessed following vaginal delivery.
Pregnancy : Category C
Carbetocin is not indicated during pregnancy, prior to the delivery of the infant. Use of carbetocin during pregnancy could result in hyperstimulation of the uterus with hypertonic or tetanic contractions, tumultuous labour, uterine rupture, cervical and vaginal lacerations, postpartum haemorrhage, uteroplacental hypoperfusion and deceleration of the foetal heart rate, foetal hypoxia, hypercapnea, or death.
Lactation :
Carbetocin, in small amounts, has been distributed in the breast milk. This small amount found in breast milk or colostrum after a single injection would not be expected to present a significant safety concern. There is insufficient evidence to determine whether carbetocin stimulates milk let-down. However, normal let-down occurred in five nursing mothers who received 70 mcg of CARBETOCIN INJECTION intramuscularly.
Paediatric Use :
No information is available on the relationship of age to the effects of CARBETOCIN INJECTION in paediatric patients. Safety and efficacy have not been established.
INTERACTIONS :
Specific interaction studies have not been undertaken. Since carbetocin is closely related in structure to oxytocin, the occurrence of interactions known to be associated with oxytocin cannot be excluded : severe hypertension has been reported when oxytocin was given 3 to 4 hours following prophylactic administration of a vasoconstrictor in conjunction with caudal-block anaesthesia. During combination with ergot-alkaloids, such as methylergometrine, oxytocin and carbetocin may enhance the blood pressure enhancing effect of these agents. If oxytocin or methylergometrine are administered after carbetocin there may be a risk of cumulative effect. Some inhalation-anaesthetics, such as halothane and cyclopropane may enhance the hypotensive effect and weaken the effect of carbetocin on the uterus.
Arrhythmias have been reported for oxytocin during concomitant use.
INCOMPATIBILITIES :
CARBETOCIN INJECTION should be given by I.V. bolus and should not be mixed with other infusion fluids.
SIDE EFFECTS :
Nausea, vomiting, abdominal pain, metallic taste, flushing, hypotension, chest pain, dyspnoea, headache, tremor, dizziness, anaemia, back pain, pruritus, feeling of warmth, chills, tachycardia and sweating also reported.
OVERDOSAGE :
Overdosage of CARBETOCIN INJECTION may produce uterine hyperactivity whether or not due to hypersensitivity to this agent. Hyperstimulation with strong (hypertonic) or prolonged (tetanic) contractions resulting from oxytocin overdose can lead to uterine rupture or postpartum haemorrhage. Overdosage of oxytocin may lead to hyponatraemia and water intoxication in severe cases, especially when associated with excessive concomitant fluid intake. As CARBETOCIN INJECTION is an analogue of oxytocin, the possibility of a similar event cannot be excluded.
TREATMENT OF OVERDOSAGE :
Treatment of overdosage of CARBETOCIN INJECTION consists of symptomatic and supportive therapy. When signs or symptoms of overdosage occur oxygen should be given to the mother. In cases of water intoxication it is essential to restrict fluid intake, promote diuresis, correct electrolyte imbalance, and control convulsions that may eventually occur.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store in refrigerator between 2°C to 8°C (36°F to 46°F), protected from light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CARBETOCIN INJECTION is supplied as 100 mcg of Carbetocin in 1 ml ampoule.
Single Ampoule per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular