333 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
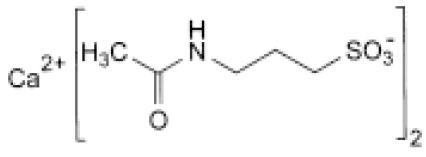
ACAMPROSATE CALCIUM TABLETS (Acamprosate Calcium) is the calcium salt to prevent relapse in alcoholics who have been weaned off alcohol. Chemically, Acamprosate Calcium is Calcium bis[3-(acetylamino)propane-1-sulfonate]. The molecular formula is C10H20CaN2O8S2 and molecular weight is 400.5.
STRUCTURAL FORMULA :
Its structural formula is :
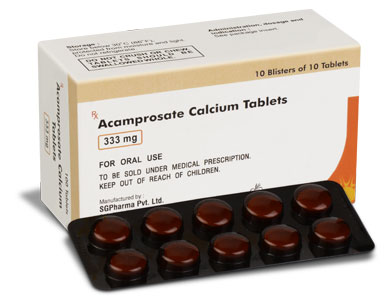
ACAMPROSATE CALCIUM TABLETS are white coloured, circular, biconvex, enteric-coated tablets plain on both side.
COMPOSITION :
Each enteric-coated tablet contains :
Acamprosate Calcium B.P. 333 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTIONS :
The mechanism of action of acamprosate in maintenance of alcohol abstinence is not completely understood. Chronic alcohol exposure is hypothesized to alter the normal balance between neuronal excitation and inhibition. In vitro and in vivo studies in animals have provided evidence to suggest acamprosate may interact with glutamate and GABA neurotransmitter systems centrally, and has led to the hypothesis that acamprosate restores this balance. Pharmacodynamic studies have shown that acamprosate calcium reduces alcohol intake in alcohol-dependent animals in a dose-dependent manner and that this effect appears to be specific to alcohol and the mechanisms of alcohol dependence. Acamprosate calcium has negligible observable central nervous system (CNS) activity in animals outside of its effects on alcohol dependence, exhibiting no anticonvulsant, antidepressant, or anxiolytic activity. The administration of acamprosate calcium is not associated
with the development of tolerance or dependence in animal studies. Acamprosate Calcium is not known to cause alcohol aversion and does not cause a disulfiram-like reaction as a result of ethanol ingestion.
PHARMACOKINETICS :
Absorption :
The absolute bioavailability of Acamprosate Calcium after oral administration is about 11 %. Steady-state plasma concentrations of acamprosate are reached within 5 days of dosing. Steady-state peak plasma concentrations after Acamprosate Calcium doses of 2 x 333 mg tablets three times daily average 350 ng/ml and occur at 3 - 8 hours post-dose. Coadministration of Acamprosate Calcium with food decreases bioavailability as measured by Cmax and AUC, by approximately 42 % and 23 %, respectively. The food effect on
absorption is not clinically significant and no adjustment of dose is necessary.
Distribution :
The volume of distribution for acamprosate following intravenous administration is estimated to be 72 - 109 litres (approximately 1 l/kg). Plasma protein binding of acamprosate is negligible.
Metabolism :
Acamprosate does not undergo metabolism.
Elimination :
After oral dosing of 2 x 333 mg of Acamprosate Calcium, the terminal half-life ranges from approximately 20 - 33 hours. Following oral administration of Acamprosate Calcium, the major route of excretion is via the kidneys as acamprosate.
Special Populations
Gender :
Acamprosate Calcium does not exhibit any significant pharmacokinetic differences between male and female subjects.
Age :
The pharmacokinetics of Acamprosate Calcium have not been evaluated in a geriatric population. However, since renal function diminishes in elderly patients and acamprosate is excreted unchanged in urine, acamprosate plasma concentrations are likely to be higher in the elderly population compared to younger adults.
Paediatrics :
The pharmacokinetics of Acamprosate Calcium have not been evaluated in a paediatric population.
Renal Impairment :
Peak plasma concentrations after administration of a single dose of 2 x 333 mg Acamprosate Calcium tablets to patients with moderate or severe renal impairment were about 2 - fold and 4 - fold higher, respectively, compared to healthy subjects. Similarly,elimination
half-life was about 1.8 - fold and 2.6 - fold longer, respectively, compared to healthy subjects. There is a linear relationship between creatinine clearance values and total apparent plasma clearance, renal clearance and plasma half-life of acamprosate. A dose of 1 x 333 mg Acamprosate Calcium, three times daily, is recommended in patients with moderate renal impairment (creatinine clearance of 30 - 50 ml/min. Acamprosate Calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤ 30 ml/min)
Hepatic Impairment :
Acamprosate is not metabolized by the liver and the pharmacokinetics of Acamprosate Calcium are not altered in patients with mild to moderate hepatic impairment (groups A and B of the Child-Pugh classification). No adjustment of dosage is recommended in such patients.
Alcohol-dependent subjects :
A cross-study comparison of Acamprosate Calcium at doses of 2 x 333 mg three times daily indicated similar pharmacokinetics between alcohol-dependent subjects and healthy subjects.
INDICATIONS :
ACAMPROSATE CALCIUM TABLETS is indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation. Treatment with Acamprosate Calcium should be part of a comprehensive management program that includes psychosocial support. The efficacy of ACAMPROSATE CALCIUM TABLETS in promoting abstinence has not been demonstrated in subjects who have not undergone detoxification and not achieved alcohol abstinence prior to beginning Acamprosate Calcium treatment. The efficacy of Acamprosate Calcium in promoting abstinence from alcohol in polysubstance abusers has not been adequately assessed.
Administration :
For Oral Use. Do not crush or chew. Tablets should be swallowed whole.
Dosage :
Adults within the age range 18 - 65 years :
2 tablets three times daily with meals (2 tablets morning, noon and night) in subjects weighing 60 kg or more.
In subjects weighing less than 60 kg, 4 tablets divided into three daily doses with meals (2 tablets in the morning, 1 at noon and 1 at night).
Children and the Elderly :
ACAMPROSATE CALCIUM TABLETS should not be administered to children and the elderly. The recommended treatment period is one year. Treatment with ACAMPROSATE CALCIUM TABLETS should be initiated as soon as possible, after the withdrawal period and should be maintained if the patient relapses. Acamprosate does not prevent the harmful effects of continuous alcohol abuse. Continued alcohol abuse negates the therapeutic benefit; therefore acamprosate treatment should only be initiated after weaning therapy, once the patient is abstinent from alcohol.
Dosage in Renal Impairment :
For patients with moderate renal impairment (creatinine clearance of 30 - 50 ml/minute), a starting dose of one 333 mg tablet taken three times daily is recommended. Acamprosate Calcium is contraindicated in patients with severe renal impairment (creatinine clearance of ≤ 30 ml/min).
CONTRAINDICATIONS :
ACAMPROSATE CALCIUM TABLETS is contraindicated in patients who previously have exhibited hypersensitivity to acamprosate calcium or any of its components. ACAMPROSATE CALCIUM TABLETS is contraindicated in patients with severe renal impairment (creatinine clearance </ = 30 ml/minute).
WARNINGS AND PRECAUTIONS :
The safety and efficacy of ACAMPROSATE CALCIUM TABLETS has not been established in patients younger than 18 years or older than 65 years. ACAMPROSATE CALCIUM TABLETS is therefore not recommended for use in these populations. The safety and efficacy of ACAMPROSATE CALCIUM TABLETS has not been established in patients with severe liver insufficiency (Childs-Pugh Classification C). Because the interrelationship between alcohol dependence, depression and suicidality is well-recognised and complex, it is
recommended that alcohol-dependent patients, including those treated with acamprosate, be monitored for such symptoms.
Abuse and dependence
Non-clinical studies suggest that acamprosate has little or no abuse potential. No evidence of dependence on acamprosate was found in any clinical study thus demonstrating that acamprosate has no significant dependence potential.
Pregnancy :
Pregnancy Category C.
There is no adequate data from the use of acamprosate calcium in pregnant women. Animal studies do not indicate any evidence of foetotoxicity or tetragenicity. ACAMPROSATE CALCIUM TABLETS must therefore only be used during pregnancy after a careful benefit/risk assessment, when the patient cannot abstain from drinking alcohol without being treated with acamprosate and when there is consequently a risk of foetotoxicity or teratogenicity due to alcohol.
Nursing mothers :
It is known that acamprosate calcium is excreted in the milk of lactating animals. It is not known whether acamprosate is excreted in human milk. There are no adequate data from the use of acamprosate in infants. ACAMPROSATE CALCIUM TABLETS must therefore not be used in breastfeeding women. If a breastfeeding woman cannot abstain from drinking alcohol without being treated with acamprosate, a decision must be made whether to discontinue nursing or to discontinue ACAMPROSATE CALCIUM TABLETS, taking into account the importance of the medicinal product to the woman.
Paediatric Use :
The safety and efficacy of acamprosate calcium have not been established in the paediatric population.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
ACAMPROSATE CALCIUM TABLETS has no influence on the ability to drive and use machines.
INTERACTIONS AND INCOMPATIBILITIES :
The concomitant intake of alcohol and acamprosate does not affect the pharmacokinetics of either alcohol or acamprosate. Administering acamprosate with food diminishes the bioavailability of the drug compared with its administration in the fasting state. In clinical trials, acamprosate has been safely administered in combination with antidepressants, anxiolytics, hypnotics and sedatives, and non-opioid analgesics Pharmacokinetic studies have been completed and show no interaction between acamprosate and diazepam, disulfiram,
oxazepam, tetrabamate, meprobamate or imipramine. There is no information available on the concomitant administration of acamprosate with diuretics.
SIDE EFFECTS :
The following definitions apply to the frequency terminology used hereafter : very common (≥ 1/10), common (≥1/100, < 1/10), uncommon (≥ 1/1,000, < 1/100), rare (≥ 1/10,000, < 1/1,000), very rare (< 1/10,000, including isolated cases), frequency not known (cannot be estimated from the available data).
Gastrointestinal disorders :
Very common : Diarrhoea
Common : Abdominal pain, nausea, vomiting, flatulence.
Skin and subcutaneous tissue disorders :
Common : Pruritus, maculo-papular rash.
Not known : Vesiculo-bullous eruptions.
Immune system disorders :
Very rare : Hypersensitivity reactions including urticaria,
angio-oedema or anaphylactic reactions.
Reproductive system and breast disorders :
Common : Frigidity or impotence.
Psychiatric disorders :
Common : Decreased libido.
Uncommon : Increased libido.
INFORMATION FOR PATIENTS :
Physicians are advised to discuss the following issues with patients for whom they prescribe ACAMPROSATE CALCIUM TABLETS.
Renal Impairment :
A lower dose is recommended for patients with moderate renal impairment. ACAMPROSATE CALCIUM TABLETS is contraindicated in patients with severe renal impairment (creatine clearance of ≤ 30 ml/minute).
Suicidality and Depression :
Families and caregivers of patients being treated with ACAMPROSATE CALCIUM TABLETS should be alerted to the need to monitor patients for the emergence of symptoms of depression or suicidality, and to report such symptoms to the patient’s health care provider
Alcohol Withdrawal :
Use of ACAMPROSATE CALCIUM TABLETS does not eliminate or diminish withdrawal symptoms .
Pregnancy and Breast Feeding :
• Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy.
• Advise patients to notify their physician if they are breast-feeding.
Relapse to Drinking :
• Advise patients to continue ACAMPROSATE CALCIUM TABLETS therapy as directed, even in the event of relapse and remind them to discuss any renewed drinking with their physicians.
• Advise patients that ACAMPROSATE CALCIUM
TABLETS has been shown to help maintain abstinence only when used as a part of a treatment program that includes counselling and support.
OVERDOSAGE :
In all reported cases of acute overdosage with Acamprosate Calcium (total reported doses of up to 56 grams of acamprosate calcium), the only symptom that could be reasonably associated with Acamprosate Calcium was diarrhoea. Hypocalcaemia has not been reported in cases of acute overdose. A risk of hypocalcaemia should be considered in chronic overdosage only.
TREATMENT OF OVERDOSAGE :
Treatment of overdose should be symptomatic and supportive.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 Months from the date of manufacture.
PRESENTATION :
ACAMPROSATE CALCIUM TABLETS contains Acamprosate Calcium B.P. 333 mg.
10 Blisters of 10 Tablets per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular