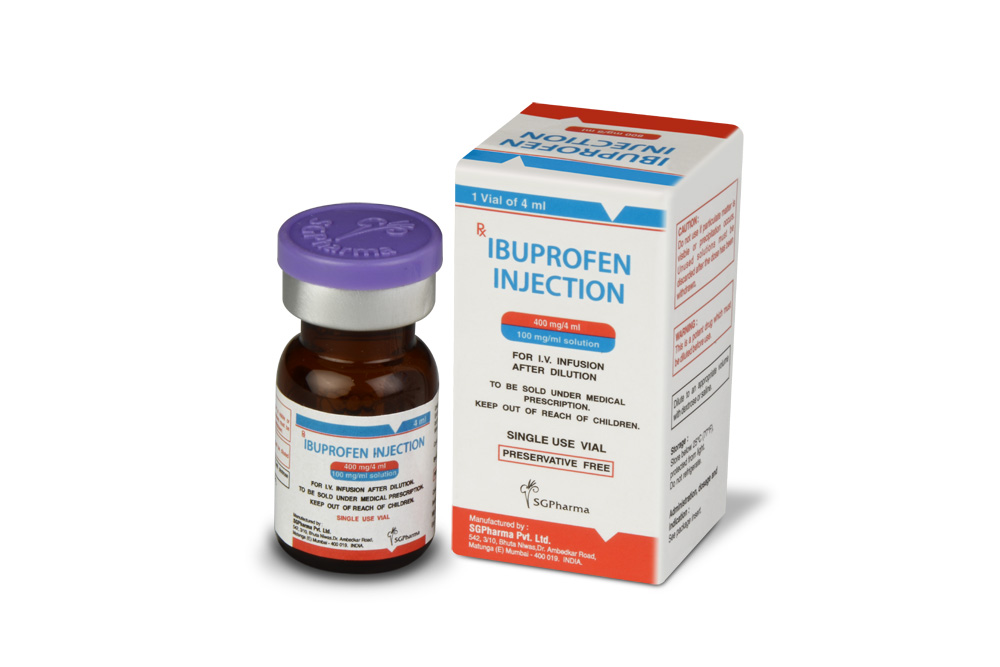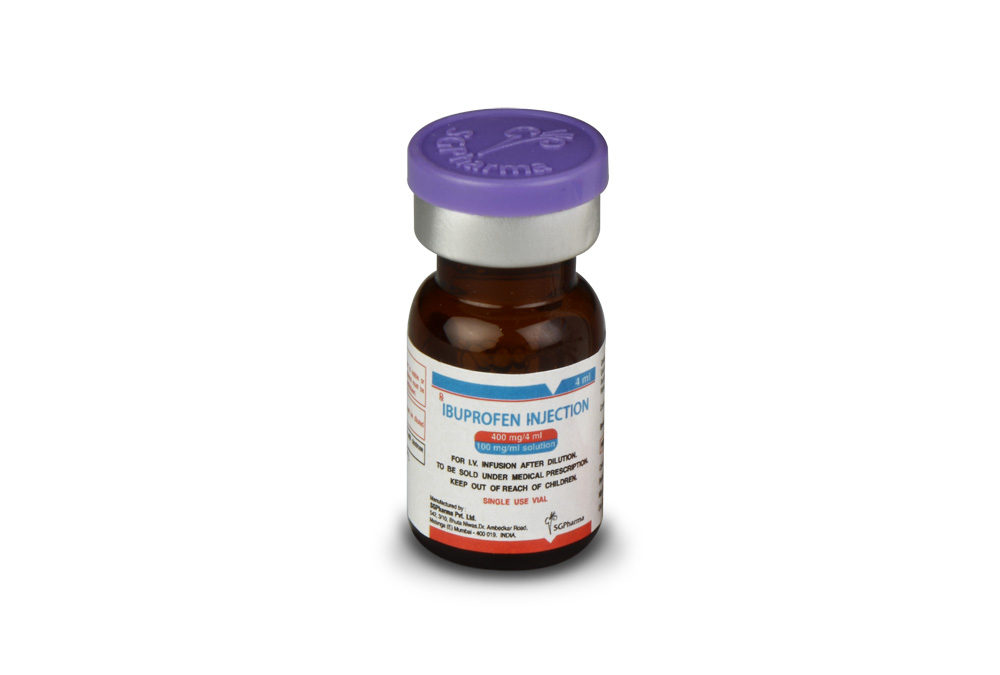
400 mg/4 ml, 800 mg/8 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
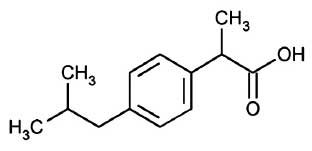
IBUPROFEN INJECTION is a non-steroidal anti-inflammatory drug (NSAID). Chemically, IBUPROFEN is (±)-2-(p-Isobutylphenyl)propionic acid. The molecular formula is C13H18O2 and molecular weight is 206.28.
STRUCTURAL FORMULA :
Its structural formula is :
IBUPROFEN INJECTION is a clear, colourless to pale yellow, sterile solution filled in amber tubular vial of suitable size.
COMPOSITION :
Each ml contains :
Ibuprofen USP 100 mg
Water for Injection USP q.s.
ACTIONS :
Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) that possesses anti-inflammatory, analgesic and antipyretic activity. Its mode of action, like that of other NSAIDs, is not completely understood, but may be related to prostaglandin synthetase inhibition.
PHARMACOKINETICS :
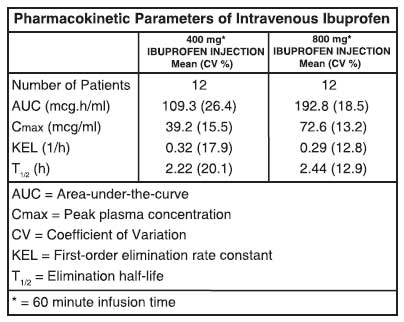
Ibuprofen is a racemic mixture of [-]R- and [+]S-isomers. In vivo and in vitro studies indicate that the [+]S-isomer is responsible for clinical activity. The [-]R-form, while thought to be pharmacologically inactive, is slowly and incompletely (~60 %) interconverted into the active [+]S species in adults. The degree of interconversion in children is unknown, but is thought to be similar. The [-]R-isomer serves as a circulating reservoir to maintain levels of active drug. The pharmacokinetic parameters of IBUPROFEN INJECTION determined in a study with volunteers are presented below.
Ibuprofen, like most NSAIDs, is highly protein bound (> 99 % bound at 20 mcg/ml). Protein binding is saturable, and at concentrations > 20 mcg/ml binding is nonlinear. Based on oral dosing data, there is an age- or fever-related change in volume of distribution for ibuprofen.
INDICATIONS :
Analgesia :
IBUPROFEN INJECTION is indicated in adults for the management of mild to moderate pain and the management of moderate to severe pain as an adjunct to opioid analgesics.
Antipyretic :
IBUPROFEN INJECTION is indicated for the reduction of fever in adults.
Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. After observing the response to initial therapy with IBUPROFEN INJECTION, the dose and frequency should be adjusted to suit an individual patient’s needs. Do not exceed 3200 mg total daily dose. To reduce the risk of renal adverse reactions, patients must be well hydrated prior to administration of IBUPROFEN INJECTION.
Analgesia :
Administer 400 mg to 800 mg intravenously every 6 hours as necessary. Infusion time must be no less than 30 minutes.
Antipyretic :
Administer 400 mg intravenously, followed by 400 mg every 4 to 6 hours or 100-200 mg every 4 hours as necessary. Infusion time must be no less than 30 minutes.
Preparation and Administration :
IBUPROFEN INJECTION must be diluted prior to intravenous infusion. Dilute to a final concentration of 4 mg/ml or less. Appropriate diluents include 0.9 % Sodium Chloride Injection USP (normal saline), 5 % Dextrose Injection USP (D5W), or Lactated Ringers Solution.
- 800 mg dose : Dilute 8 ml of IBUPROFEN INJECTION in not less than 200 ml of diluent.
- 400 mg dose : Dilute 4 ml of IBUPROFEN INJECTION in not less than 100 ml of diluent.
Diluted solutions are stable for up to 24 hours at ambient temperature (approximately 20 to 25°C) and room lighting. Infusion time must be not less than 30 minutes.
CONTRAINDICATIONS :
Hypersensitivity :
IBUPROFEN INJECTION is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to Ibuprofen.
Asthma and Allergic Reactions :
IBUPROFEN INJECTION is contraindicated in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal anaphylactic-like reactions to NSAIDs have been reported in such patients.
Coronary Artery Bypass Graft (CABG) :
IBUPROFEN INJECTION is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.
WARNINGS AND PRECAUTIONS :
Cardiovascular Thrombotic Events :
Non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
Gastrointestinal Effects (Risk of Ulceration, Bleeding, and Perforation) :
NSAIDs, including Ibuprofen, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1 % of patients treated for 3-6 months and in about 2-4 % of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short term therapy is not without risk. Prescribe IBUPROFFEN INJECTION with extreme caution in those with a prior history of ulcer disease or GI bleeding. Patients with a prior history of peptic ulcer disease and/or GI bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to treated patients with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most reports of spontaneous fatal GI events are in elderly or debilitated patients, and therefore special care should be taken in treating this population.
Hepatic Effects :
Borderline elevations of one or more liver tests may occur in up to 15 % of patients taking NSAIDs, including ibuprofen. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately 3 or more times the upper limit of normal) have been reported in approximately 1 % of patients. In addition, rare cases of severe hepatic reactions have been reported, including jaundice, fulminant hepatitis, liver necrosis and hepatic failure, some with fatal outcomes. A patient with symptoms and/or signs suggesting liver dysfunction, or with abnormal liver test values, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with ibuprofen. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), ibuprofen should be discontinued.
Hypertension :
NSAIDs, including ibuprofen, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Use NSAIDs, including ibuprofen, with caution in patients with hypertension. Monitor blood pressure closely during the initiation of NSAID treatment and throughout the course of therapy. Patients taking ACE inhibitors, thiazides, or loop diuretics may have impaired response to these therapies when taking NSAIDs.
Renal Effects :
Use caution when initiating treatment with IBUPROFEN INJECTION in patients with considerable dehydration. Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics or ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pre-treatment state. No information is available from controlled clinical studies regarding the use of IBUPROFEN INJECTION in patients with advanced renal disease. If IBUPROFEN INJECTION therapy must be initiated in patients with advanced renal disease, closely monitor the patient’s renal function.
Anaphylactic Reactions :
As with other NSAIDs, anaphylactic reactions may occur in patients without known prior exposure to ibuprofen. IBUPROFEN INJECTION is contraindicated in patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs.
Serious Skin Reactions :
NSAIDs, including ibuprofen, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens - Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin manifestations, and to discontinue IBUPROFEN INJECTION at the first appearance of skin rash or any other sign of hypersensitivity.
Masking Inflammation and Fever :
The pharmacological activity of ibuprofen in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed non-infectious, painful conditions.
Haematological Effects :
IBUPROFEN INJECTION must be diluted prior to use. Infusion of the drug product without dilution can cause haemolysis. Anaemia may occur in patients receiving NSAIDs, including ibuprofen. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect on erythropoiesis. In patients on long-term treatment with NSAIDs, including ibuprofen, check haemoglobin or haematocrit if they exhibit any signs or symptoms of anaemia or blood loss. NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effects on platelet function are less severe quantitatively, of shorter duration, and reversible. Carefully monitor patients who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants.
Pre-existing Asthma :
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross-reactivity between aspirin and NSAIDs has been reported in such aspirin-sensitive patients, including bronchospasm, IBUPROFEN INJECTION is contraindicated in patients with this form of aspirin sensitivity and should be used with caution in all patients with pre-existing asthma.
Ophthalmological Effects :
Blurred or diminished vision, scotomata, and changes in colour vision have been reported with oral ibuprofen. Discontinue ibuprofen if a patient develops such complaints, and refer the patient for an ophthalmologic examination that includes central visual fields and colour vision testing.
Aseptic Meningitis :
Aseptic meningitis with fever and coma has been observed in patients on oral ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have underlying chronic disease. If signs or symptoms of meningitis develop in a patient on ibuprofen, give consideration to whether or not the signs or symptoms are related to ibuprofen therapy.
Monitoring :
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have CBC and chemistry profiles checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash), or abnormal liver tests persist or worsen, discontinue IBUPROFEN INJECTION.
Pregnancy :
Teratogenic effects - Category C prior to 30 weeks gestation; Category D starting at 30 weeks gestation Starting at 30 weeks gestation, IBUPROFEN INJECTION, and other NSAIDs, should be avoided by pregnant women as premature closure of the ductus arteriosus in the foetus may occur. IBUPROFEN INJECTION can cause foetal harm when administered to a pregnant woman starting at 30 weeks gestation. There are no adequate, well-controlled studies in pregnant women. Prior to 30 weeks gestation, IBUPROFEN INJECTION should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus. Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities.
Labor and Delivery :
The effects of IBUPROFEN INJECTION on labor and delivery in pregnant women are unknown. In rat studies, maternal exposure to NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, increased the incidence of dystocia and delayed parturition, and decreased pup survival.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from IBUPROFEN INJECTION, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Use :
Safety and effectiveness of IBUPROFEN INJECTION for management of pain and reduction of fever has not been established in paediatric patients below the age of 17 years.
Geriatric Use :
Clinical studies of IBUPROFEN INJECTION did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients are at increased risk for serious GI adverse events.
INTERACTIONS AND INCOMPATIBILITIES :
Aspirin :
When ibuprofen is administered with aspirin, ibuprofen’s protein binding is reduced, although the clearance of free ibuprofen is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of IBUPROFEN INJECTION and aspirin is not generally recommended because of the potential for increased adverse effects.
Anticoagulants :
The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that the users of both drugs together have a higher risk of serious GI bleeding than users of either drug alone.
ACE Inhibitors :
NSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors.
Diuretics :
Clinical studies and post-marketing observations have shown that ibuprofen can reduce the natriuretic effects of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, observe patients closely for signs of renal failure, as well as to assure diuretic efficacy.
Lithium :
NSAIDs have produced elevations of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15 %, and the renal clearance of lithium decreased by 20 %. This effect has been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, observe patients carefully for signs of lithium toxicity.
Methotrexate :
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This indicates that NSAIDs may enhance the toxicity of methotrexate. Use caution when NSAIDs are administered concomitantly with methotrexate.
H2 Antagonist :
In studies of human volunteers, co-administration of cimetidine or ranitidine with ibuprofen had no substantive effect on ibuprofen serum concentrations.
SIDE EFFECTS :
The following serious adverse reactions are discussed in WARNINGS AND PRECAUTIONS :
- Cardiovascular thrombotic events
- Gastrointestinal effects
- Hepatic effects
- Hypertension
- Congestive heart failure and oedema
- Renal effects
- Anaphylactoid reactions
- Serious skin reactions
The most common adverse reactions reported in clinical studies are nausea, flatulence, vomiting, and headache. The most common reason for discontinuation due to adverse events in controlled trials of IBUPROFEN INJECTION is pruritus (< 1 %).
INFORMATION FOR PATIENTS :
Patients should be informed of the following information before initiating therapy with an NSAID.
Cardiovascular Effects :
Ibuprofen, like other NSAIDs, may cause serious CV events such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, advise patients to be alert for the signs and symptoms of chest pain, shortness of breath, weakness, and slurring of speech, and to ask for medical advice when observing any indicative sign or symptoms. Inform patients of the importance of this follow-up.
Gastrointestinal Effects :
Ibuprofen, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, advise patients to be alert for the signs and symptoms of ulcerations and bleeding, and to ask for medical advice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and haematemesis. Inform patients of the importance of this follow-up.
Hepatotoxicity :
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flulike” symptoms). Instruct patients to stop therapy with IBUPROFEN INJECTION and seek immediate medical therapy if any of these occur.
Adverse Skin Reactions :
Ibuprofen, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, advise patients to be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and to ask for medical advice when observing any indicative sign or symptoms. Advice patients to stop IBUPROFEN INJECTION immediately if they develop any type of rash and to contact a physician as soon as possible.
Weight Gain and Oedema :
Advice patients to promptly report to their physicians signs or symptoms of unexplained weight gain or oedema during treatment with IBUPROFEN INJECTION.
Anaphylactoid Reactions :
Inform patients of the signs of an anaphylactoid reaction (e.g. difficulty in breathing, swelling of the face or throat). If these occur, therapy should be discontinued and medical therapy initiated.
Effects During Pregnancy :
Starting at 30 weeks gestation, IBUPROFEN INJECTION and other NSAIDs should be avoided by pregnant women as premature closure of the ductus arteriosus in the foetus may occur.
OVERDOSAGE :
The following signs and symptoms have occurred in individuals following an overdose of oral ibuprofen : abdominal pain, nausea, vomiting, drowsiness, and dizziness.
TREATMENT OF OVERDOSAGE :
There are no specific measures to treat acute overdosage with IBUPROFEN INJECTION. There is no known antidote to ibuprofen. In case of an overdosage, discontinue IBUPROFEN INJECTION therapy.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
IBUPROFEN INJECTION is supplied as below :

Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular