125 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Hydrocortisone Acetate is a synthetic adrenocortical steroid. Chemically, Hydrocortisone Acetate is 11ß, 17-dihydroxy-3, 20-dioxopregn-4-en-21-yl acetate, the molecular formula is C23H32O6 and molecular weight is 404.5.
STRUCTURAL FORMULA :
Its structural formula is :
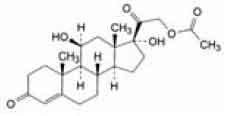
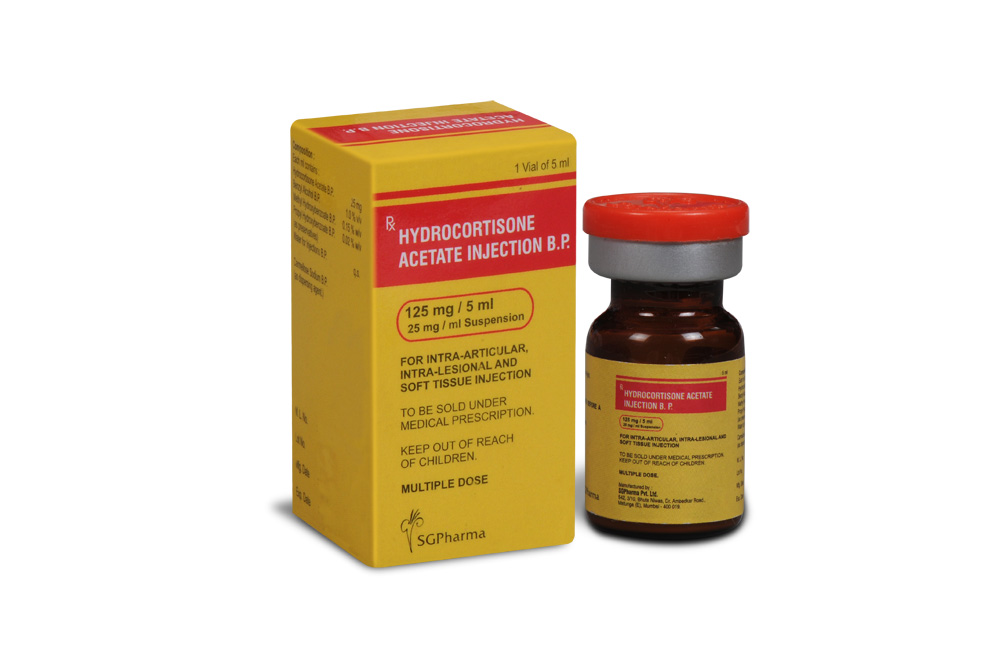
Hydrocortisone Acetate Injection B.P. is a sterile aqueous suspension in water for injection, filled in vial of suitable size.
COMPOSITION :
Each ml contains :
Hydrocortisone Acetate B.P. 25 mg
Benzyl Alcohol B.P. 1.0 % w/v
Methyl Hydroxybenzoate B.P. 0.15 % w/v
Propyl Hydroxybenzoate B.P. 0.02 % w/v
(As preservatives)
Water for Injections B.P. q.s.
Carmellose Sodium B.P.
As dispersing agent)
ACTIONS :
Hydrocortisone Acetate has anti-inflammatory and immunosuppressive action. It alters the immune response due to its effects on lymphocyte number and inhibition of their functions. Hydrocortisone Acetate Injection B.P. has a slow onset but long duration of action when compared with more soluble preparations. Because of its insolubility, it is suitable for intra-articular, intralesional, and soft tissue injection where its anti-inflammatory effects are confined mainly to the area in which it has been injected, although it is capable of producing systemic hormonal effects like altering carbohydrate, lipid and protein metabolism.
PHARMACOKINETICS :
The plasma half-life is about 100 minutes. It is more than 90 % bound to plasma proteins. Absorption of hydrocortisone acetate after intra-articular or soft tissue injection is slow but complete. Hydrocortisone is metabolised in the liver and most body tissues to hydrogenated and degraded forms such as tetrahydrocortisone and tetrahydrocortisol. These are excreted in the urine, mainly conjugated as glucuronides, together with a very small proportion of unchanged hydrocortisone. Hydrocortisone readily crosses the placenta.
INDICATIONS :
A. By intra-articular or soft tissue injection : As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in :
Synovitis of osteoarthritis
Rheumatoid arthritis
Acute and subacute bursitis
Acute gouty arthritis
Epicondylitis
Acute nonspecific tenosynovitis
Post-traumatic osteoarthritis, Psoriatic arthritis.
B. By intralesional injection :
Keloids
Localized hypertrophic, infiltrated, inflammatory lesions of : lichen planus, psoriatic plaques, granuloma annulare, and lichen simplex chronicus (neurodermatitis) Discoid lupus erythematosus Necrobiosis lipoidica diabeticorum
Alopecia areata
May also be useful in cystic tumors of an aponeurosis or tendon (ganglia).
Administration :
For Intra-articular, intralesional and soft tissue injection only.
FOR LOCAL ACTION ONLY.
VIAL SHOULD BE GENTLY SHAKEN BEFORE
A DOSE IS WITHDRAWN.
Dosage :
DOSAGE AND FREQUENCY OF INJECTION ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE AND THE RESPONSE OF THE PATIENT. The initial dose varies from 5 to 75 mg depending on the disease being treated and the size of the area to be injected. Frequency of injection depends on symptomatic response and usually is once every two or three weeks. Severe conditions may require injection once a week. Frequent intra-articular injection may result in damage to joint tissues. If satisfactory clinical response does not occur after a reasonable period of time, discontinue Hydrocortisone Acetate injection B.P. and transfer the patient to other therapy. Patients should be observed closely for signs that might require dosage adjustment, including changes in clinical status resulting from remissions or exacerbations of the disease and individual drug responsiveness.
immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis. Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Intra-articular injection of a corticosteroid may produce systemic as well as local effects. Appropriate examination of any joint fluid present is necessary to exclude a septic process. Corticosteroids should not be injected into unstable joints.A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever and malaise is suggestive of septic arthritis. If this complication occurs and the diagnosis of sepsis is confirmed, appropriate antimicrobial therapy should be instituted. Patients should be impressed strongly with the importance of not overusing joints in which symptomatic benefit has been obtained as long as the inflammatory process remains active. Frequent intra-articular Injection may result in damage to joint tissues.
Pregnancy : Pregnancy Category C :
Since adequate human reproduction studies have not been done with corticosteroids, use of these drugs in pregnancy or in women of childbearing potential requires that the anticipated benefits be weighed against the possible hazards to the mother and embryo or foetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing mothers :
Corticosteroids appear in breast milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other unwanted effects. Mothers taking pharmacologic doses of corticosteroids should be advised not to nurse.
Paediatric Use :
hould be cautiously used in Paediatric patients. Growth and development of paediatric patients on prolonged corticosteroid therapy should be carefully followed.
INTERACTIONS AND INCOMPATIBILITIES :
se of following drugs together with hydrocortisone acetate may accelerate its excertion :
Antiepileptics eg : carbamazepine, phenytoin.
Barbiturates eg : Phenobarbitone.
Rifampicin.
Aminoglutethimide.
Use of hydrocortisone acetate with diuretics like Frusemide, other medications like acetazolamide, amphotericin may increase the
risk of hypokalaemia.
Increases the risk of stomach ulceration and
bleeding when used with NSAIDs.
Live vaccines should not administer to people
taking corticosteroids.
SIDE EFFECTS :
Fluid and electrolyte disturbances :
Sodium retention.
Fluid retention.
Congestive heart failure in susceptible patients.
Potassium loss.
Hypokalaemic alkalosis.
Hypertension.
Musculoskeletal :
Muscle weakness.
Steroid myopathy.
Loss of muscle mass.
Osteoporosis.
Vertebral compression fractures.
Aseptic necrosis of femoral and humeral heads.
Pathologic fracture of long bones.|Tendon rupture.
Gastrointestinal :
Peptic ulcer with possible subsequent perforation and haemorrhage. Perforation of the small and large bowel, particularly in patients with inflammatory bowel disease.
Pancreatitis.
Abdominal distention.
Ulcerative oesophagitis.
Dermatologic :
Impaired wound healing.
Thin fragile skin.
Petechiae and ecchymoses.
Erythema.
Increased sweating.
ay suppress reactions to skin tests.
Other cutaneous reactions, such as allergic dermatitis, urticaria, angioneurotic oedema.
Neurologic :
Convulsions.
Increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatment.
Vertigo.
Headache.
Psychic disturbances.
Endocrine : Menstrual irregularities. Development of cushingoid state. Suppression of growth in children. Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness. Decreased carbohydrate tolerance.
Manifestations of latent diabetes mellitus. Increased requirements for insulin or oral hypoglycemic agents in diabetics.
Hirsutism.
Ophthalmic :
Posterior subcapsular cataracts. Increased intraocular pressure.
Glaucoma.
Exophthalmos.
Metabolic : Negative nitrogen balance due to protein catabolism.
Cardiovascular : Myocardial rupture following recent myocardial infarction.
Other : Anaphylactoid or hypersensitivity reactions. Thromboembolism. Weight gain. Increased appetite.
Nausea.
Malaise.
The following additional adverse reactions are related to Injection of corticosteroids : Rare instances of blindness associated with intralesional therapy around the face and head.Hyperpigmentation or hypopigmentation. Subcutaneous and cutaneous atrophy. Sterile abscess. Postinjection flare (following intra-articular use). Charcot-like arthropathy.
OVERDOSAGE :
Reports of acute toxicity and/or death following overdosage of glucocorticoids are rare.
TREATMENT OF OVERDOSAGE :
In the event of overdosage, no specific antidote is available; treatment is supportive and symptomatic.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial.
STORAGE :
Store below 30°C (86°F), protected from light. Avoid excessive heat. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
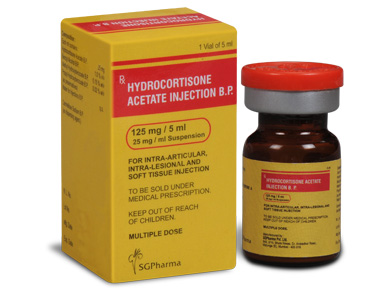
PRESENTATION :
Hydrocortisone Acetate Injection B.P. is supplied as 125 mg of Hydrocortisone Acetate B.P. in 5 ml vial.
Single vial pack.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular