250 mcg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
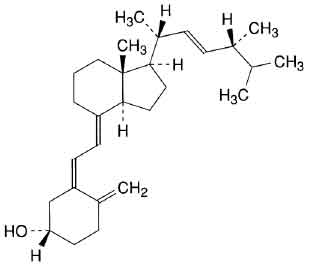
ERGOCALCIFEROL TABLETS B.P. (Ergocalciferol) is derived from fungal and plant sources. Ergocalciferol is also known as Vitamin D2. Chemically ergocalciferol is (5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3β-ol. Its molecular formula is C28H44O and its molecular weight is 396.66 daltons.
STRUCTURAL FORMULA :
Its structural formula is :
ERGOCALCIFEROL TABLETS B.P. are white coloured, circular film coated tablets.
COMPOSITION :
Each film coated tablet contains :
Ergocalciferol B.P. 10,000 IU (250 mcg)
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTIONS :
Ergocalciferol has the highest activity from the group of Vitamin D. It regulates : phosphorus-calcium metabolism and the processes of ossification ; absorption of phosphorus and amino acids in renal tubules.
PHARMACOKINETICS :
Vitamin D is absorbed in the jejunum wall and as calciferol crosses into the blood plasma and lymph. Its absorption depends on the functional condition of the pancreas and is strongly reduced in the presence of steatorrhea. Vitamin D2 and its metabolites circulates in the blood bound to a specific alpha-globulin. Vitamin D can be stored in adipose and muscle tissue for long period of time. It is slowly released from such storage sites and from the skin where it is formed in the presence of sunlight or ultraviolet light. Ergocalciferol have a slow onset and a long duration of action. The half-elimination time of Vitamin D is 26 hours. Ergocalciferol is hydroxylated in the liver by the enzyme Vitamin D 25-hydroxylase to form 25-hydroxyergocalciferol which undergo further hydroxylation in the kidneys by the enzyme Vitamin D1-hydroxylase to form the active metabolite 1, 25-dihydroxyergocalciferol. Further metabolism also occurs in the kidneys, including the formation of the 1, 24, 25-trihydroxy derivatives. The half-elimination time of this active form is between 7 and 14 days.
Vitamin D and their metabolites are excreted mainly in the bile and faeces with only small amounts appearing in urine. They also undergo extensive enterohepatic recirculation.
INDICATIONS :
ERGOCALCIFEROL TABLETS B.P. is used prophylactically. It is indicated for the treatment of refractory rickets (Vitamin D-resistant rickets), spasmophilia and osteomalacia, for acceleration of the coalescence of fractured bones, protection from dental caries, in osteoporosis, familial hypophosphataemia, hypocalcaemia associated with hypoparathyroidism, scrofula, exudative diathesis. ERGOCALCIFEROL TABLETS B.P. is also administered for the treatment of lupus, pleurisy and tuberculosis of the larynx, kidneys, etc.
Administration :
For oral use.
Dosage :
In the treatment of Vitamin D deficiency conditions 0.25 mg (10 000 units or one tablet) daily. Vitamin D deficiency caused by intestinal malabsorption or chronic liver disease usually requires doses of up to 1 mg (40,000 IU or 4 tablets) daily in divided doses. In the treatment of hypoparathyroidism 1.25 mg to 5 mg (50 000 to 200 000 units or 5 to 20 tablets) daily. The hypocalcaemia of hypoparathyroidism often requires doses of up to 5 mg (200,000 IU or 20 tablets ) daily in divided doses. Patients with renal osteodystrophy may require as much as 5 mg (200 000 units or 20 tablets) daily. For children and the elderly the adult dosage may require adjustment according to the severity of the condition.
CONTRAINDICATIONS :
Hypocalcaemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of Vitamin D, hypervitaminosis D, decreased renal function, sarcoidosis and possibly other granulomatous disease. ERGOCALCIFEROL TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
Vitamin D should be administered with caution to infants and patients who may have an increased sensitivity to its effects. Use with care in patients with renal impairment, renal calculi or heart disease or arteriosclerosis who might be at increased risk of organ damage if hypercalcaemia were to occur. All patients receiving pharmacological doses of vitamin D should have their plasma calcium concentration checked at intervals and whenever nausea and vomiting are present. Patients with rare hereditary problems of galactose intolerance, fructose intolerance, glucose-galactose malabsorption, the Lapp-lactase deficiency or sucrase-isomaltase insufficiency should not take this medicine. Adequate fluid intake should be maintained.
PRECAUTIONS :
Patients with rare hereditary problems of galactose intolerance, fructose intolerance, glucose-galactose malabsorption, the Lapp-lactase deficiency or sucrase-isomaltase insufficiency should not take this medicine. Adequate fluid intake should be maintained.
ERGOCALCIFEROL TABLETS B.P. should be used cautiously in diabetic patients.
INFORMATION FOR PATIENTS :
Importance of diet and calcium supplementation regimen adherence to achieve a clinical response to vitamin D therapy. Importance of immediate reporting to potential manifestations of hypercalcaemia. Importance of informing clinicians of existing or contemplated concomitant therapy, including prescription and OTC drugs, as well as any concomitant illnesses. Importance of women informing clinicians if they are or plan to become pregnant or plan to breast feed. Importance of informing patients of other precautionary information.
Usage in Pregnancy : Pregnancy category C
There are no adequate data on the use of Ergocalciferol in pregnant women. Ergocalciferol should not be used in pregnancy unless the potential benefit outweighs the potential hazards to the foetus. Animal studies have shown foetal abnormalities associated with hypervitaminosis D. Calcifediol and calcitriol are teratogenic in animals when given in doses several times the human dose. The offspring of a woman administered 17-144 times the recommended dose of calcitriol during pregnancy manifested mild hypercalcaemia in the first 2 days of life, which returned to normal at day 3.
Usage in Lactation :
Ergocalciferol is excreted in breast milk in limited amounts. In a mother given large doses of Ergocalciferol, 25-hydroxycholecalciferol appeared in the milk and caused hypercalcaemia in the child. Monitoring of the infants serum calcium is required in such cases. Ergocalciferol should not be administered to breast-feeding mothers.
Children :
Paediatric doses must be individualized and monitored under close medical supervision.
INTERACTIONS :
Phosphate infusions should not be administered to lower hypercalcaemia of hypervitaminosis D because of the dangers of metastatic calcification. Vitamin D requirements may be increased in patients taking anti-convulsants (e.g. carbamazepine, phenobarbital, phenytoin, and primidone). Absorption of calcium may be reduced by oral sodium sulphate or parenteral magnesium sulphate. Concurrent use of Vitamin D analogues and cardiac glycosides may result in cardiac arrhythmias due to hypercalcaemia. There is an increased risk of hypercalcaemia if vitamin D is administered with thiazide diuretics and calcium
SIDE EFFECTS :
Note : Ingestion of excessive doses of Vitamin D either as an acute overdose or over prolonged periods can result in severe toxicity. Chronic vitamin D-induced hypercalcaemia may result in generalised vascular calcification, nephrocalcinosis, and other soft tissue calcification that may lead to hypertension and renal failure. These effects are more likely to occur when the hypercalcaemia is accompanied by hyperphosphataemia. Growth may be arrested in children, especially after prolonged administration. Death may occur as a result of renal or cardiovascular failure caused by vitamin D toxicity. The toxic doses for adults amount to 1,00,000 IU daily, taken for months, in children they are between 20,000 IU and 40,000 IU daily, also after a long-term intake.
OVERDOSAGE :
Vitamin D is the most likely of all vitamins to cause overt toxicity. Infants and children are generally more susceptible to its toxic effects. Excessive intake of vitamin D leads to the development of hypercalcaemia and its associated effects including hypercalciuria, ectopic calcification, and renal and cardiovascular damage.
Hypervitaminosis D is characterized by :
Hypercalcaemia with anorexia, nausea, weakness, weight loss, vague aches and stiffness, constipation, diarrhoea, convulsions, mental retardation, anaemia, mild acidosis. Impairment of renal function with polyuria, nocturia, polydipsia, hypercalcuria, reversible azotemia, hypertension, nephrocalcinosis, generalized vascular calcification, irreversible renal insufficiency, albuminuria, or urinary casts. Widespread calcification of the soft tissues, including the heart, blood vessels, renal tubules, and lungs. Bone demineralization (osteoporosis) in adults occurs concomitantly. Decline in the average rate of linear growth and increased mineralization of bones in infants and children (dwarfism)
TREATMENT OF OVERDOSAGE :
Hypervitaminosis D is treated by withdrawal of the vitamins, low-calcium diet, and generous fluid intake. If hypercalcaemia persists, a low-calcium diet and prednisone may be started. Severe hypercalcaemia may be treated with calcitonin, etidronate, pamidronate or gallium nitrate. Hypercalcaemic crisis requires vigorous hydration with intravenous saline to increase calcium excretion, with or without a loop diuretic. Other reported therapeutic measures include dialysis or the administration of citrates, sulfates, phosphates, corticosteroids or EDTA. Cardiac arrhythmias may be treated with small doses of potassium with continuous cardiac monitoring. Therapy may be reinstituted at a lower dose when serum calcium concentrations return to normal. Serum or urinary calcium levels should be obtained twice weekly after dosage changes.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
ERGOCALCIFEROL TABLETS B.P. contains Ergocalciferol B.P. 10,000 IU (250 mcg).
250 tablets are packed in a jar.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular