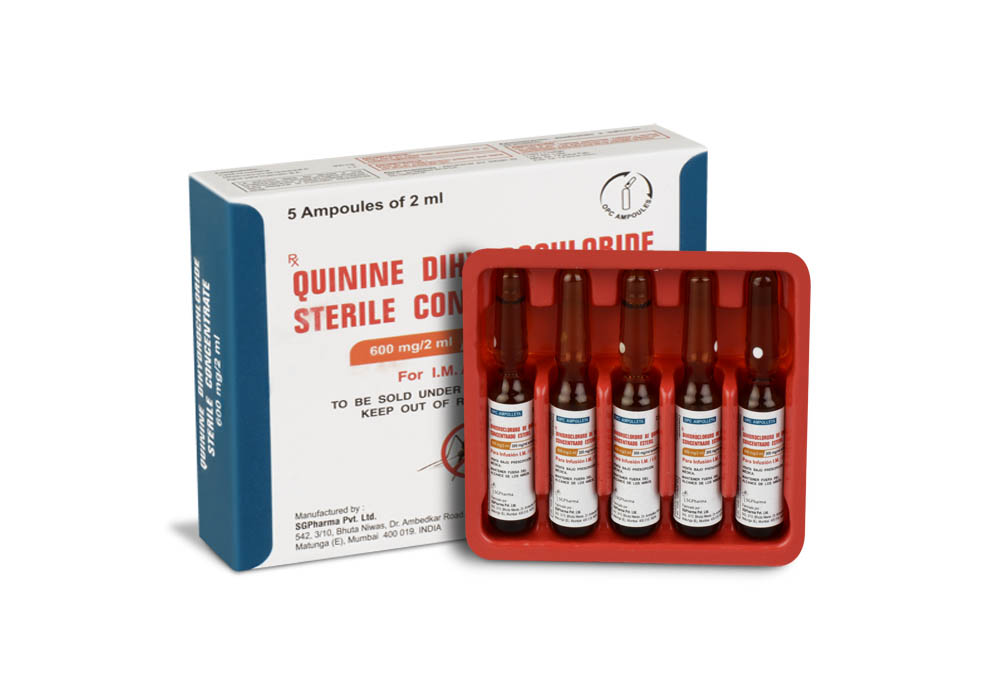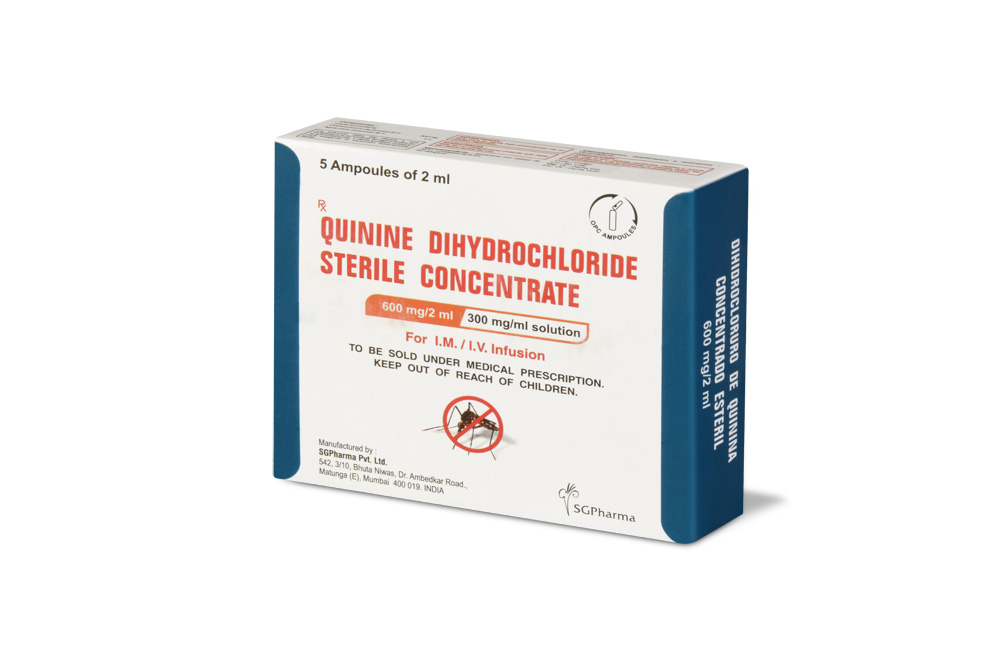
600 mg/10 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Quinine Dihydrochloride Sterile Concentrate B.P. (Quinine Dihydrochloride) is a cinchona alkaloid and a 4-methanolquinoline antimalarial that is a rapid-acting blood schizontocide with activity against Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. Chemically Quinine Dihydrochloride is (8S,9R)-6´-Methoxycinchonan-9-ol; dihydrochloride. The molecular formula is C20H24N2O2, 2HCl and molecular weight is 397.3.
STRUCTURAL FORMULA :
Its structural formula is :
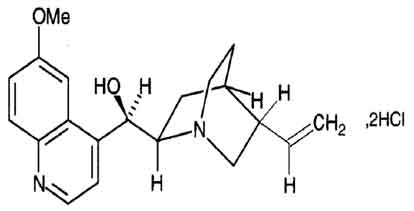
Quinine Dihydrochloride Sterile Concentrate B.P. is a clear, almost colourless to light yellow solution filled in ampoule of suitable size.
Whenever possible, patients should be transferred to oral therapy as soon as possible. For intramuscular use, refer Warnings and Precautions.
Administration :
Quinine Dihydrochloride Sterile Concentrate B.P. is for I.M. / I.V. use.
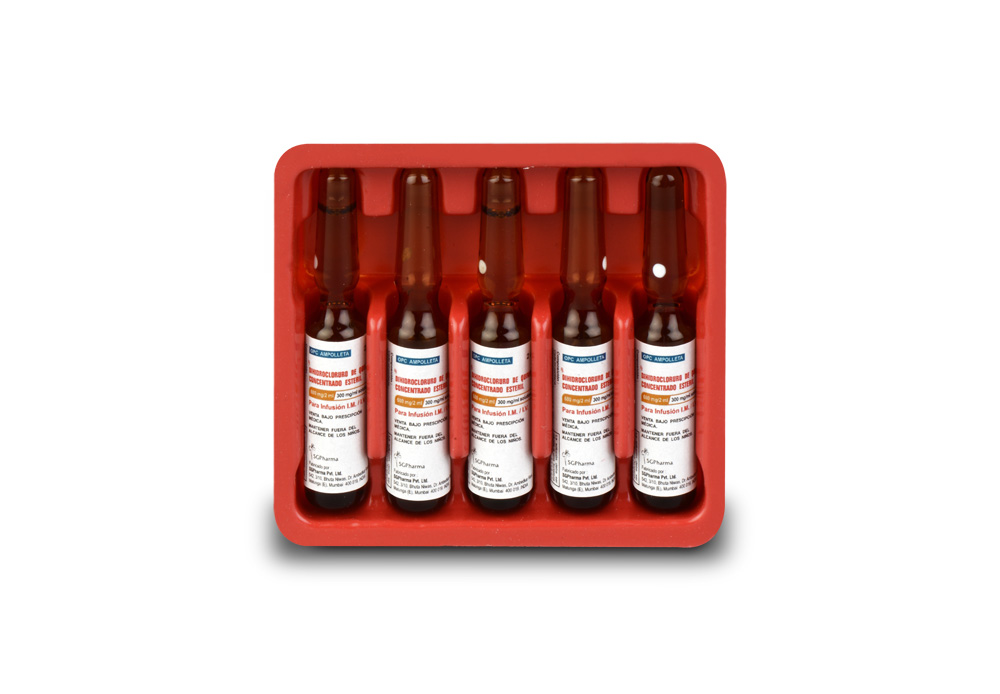
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults :
I.V. : The following doses may be used as a guideline. Quinine dihydrochloride sterile concentrate B.P. must be diluted in 500 ml sodium chloride infusion 0.9 % or glucose infusion 5 % (usually 600 mg in 500 ml) and infused slowly over 4 hours. A loading dose of 20 mg/kg up to a maximum of 1400 mg is given slowly by infusion over 4 hours. Commence the maintenance doses 8 - 12 hours after the loading dose. A loading dose is not required if anti-malarials have been given during the previous 24 hours. A maintenance dose of 10 mg/kg up to a maximum of 700 mg over 4 hours is given slowly by infusion. Repeat every 8 - 12 hours if necessary. If parenteral therapy is required for longer than 48 hours, the maximum dose of quinine should be reduced by one-third to one-half to 5 mg/kg to avoid accumulation and drug level monitoring is important. Alternatively, in intensive care units only, an initial loading dose of 7 mg/kg may be given over 30 minutes followed immediately by the first of the maintenance doses.
Paediatric :
I.V. - 10 mg/kg diluted and infused slowly at a rate not exceeding 0.5 mg/minute, preferably with ECG monitoring.
Intramuscular use :
Adults and Children :
Quinine may be given I.M. to adults or children if I.V. administration is impossible. It must be given by deep injection into the anterior thigh, using sterile equipment and following thorough disinfection of the skin area. A loading dose of 20 mg/kg is usual in adults, followed by 8-hourly maintenance doses. Young children should be given a loading dose of 15 mg/kg, followed by 10 mg/kg maintenance doses every 12 hours.
CONTRAINDICATIONS :
Quinine Dihydrochloride Sterile Concentrate B.P. is contra-indicated in the following :
• Hypersensitivity to quinine or quinidine.
• Glucose-6-phosphate dehydrogenase (G-6-PD) deficiency.
• History of Blackwater fever.
• Patients with tinnitus or optic neuritis.
• The presence of haemolysis or concurrent anticoagulant therapy.
WARNINGS & PRECAUTIONS :
Check for hypersensitivity to quinine or quinidine before administration. It is important that when given intravenously it should be given by slow infusion and the patient observed closely for signs of cardiotoxicity. All patients receiving quinine should receive cardiac monitoring. Pulse and blood pressure should be closely monitored and the rate of infusion attenuated if dysrhythmias occur, and blood glucose concentrations should be monitored. Therapy should be changed to oral administration as soon as possible. Intramuscular use not recommended. If intravenous administration is not possible, quinine dihydrochloride has been given intramuscularly. This can be an irritant, cause pain, focal necrosis and abscess formation. Fatal tetanus has developed in some patients and there have been concerns regarding its safety and efficacy. The intramuscular route should only be used as a last resort.
Haemolysis :
Quinine Dihydrochloride Sterile Concentrate B.P. should be stopped immediately and supportive measures instituted if signs of haemolysis appear. Haemolysis with a potential for haemolytic anaemia has been reported when given to patients with G-6-PD deficiency.
Prothrombin formation :
Quinine dihydrochloride is capable of causing hypoprothrombinaemia and may enhance the effect of anticoagulants.
Atrial fibrillation :
Patients with this condition should be digitalised before receiving quinine as it may otherwise cause an increase in the ventricular rate.
Hypersensitivity :
Reactions include cutaneous flushing, pruritis, rash, fever, facial oedema, GI distress, dyspnoea, tinnitus and vision impairment. The most frequently reported hypersensitivity reaction is extreme flushing of the skin with intense pruritis. If evidence of hypersensitivity occurs, quinine therapy should be discontinued.
Liver Disease :
The half-life of quinine is prolonged in moderate chronic liver disease.
Diabetes :
Quinine has the potential to cause refractory hypoglycaemia, especially when used in combination with sulphonylureas.
Driving and Using Machinery :
Quinine is likely to produce minor or moderate adverse effects on the ability to drive or use machinery.
Pregnancy : Category D
In pregnancy, the risks associated with the use of quinine should be balanced against the risks posed by the malarial infection. In cases of life threatening malaria, the risk of quinine use may be considered acceptable. In high doses, quinine causes foetal injuries in the form of deafness, development disturbances and malformations of the extremities and cranium. It has the ability to cause uterine contractions and constitutes a risk of abortion.
Nursing mothers :
Quinine is excreted in breast milk in small concentrations and caution should be exercised during breastfeeding.
INTERACTIONS :
Urinary alkalinisers such as acetazolamide, and sodium bicarbonate increase blood quinine levels by decreasing renal clearance of quinine. Urinary acidifiers such as ammonium chloride and some other drugs that decrease the pH of urine, increase the excretion of quinine, resulting in lower blood levels. Cimetidine may reduce clearance of quinine if taken concurrently. Digoxin serum levels and effects may increase if taken concurrently with quinine. Serum digoxin concentrations should be monitored and dosage adjustments made when necessary. Hypoprothrombinaemic effects may be increased when quinine is used with warfarin, coumarin or indanedione derivatives. Anti-malarials such as pyrimethamine may displace quinine from protein binding sites resulting in excessive free quinine levels and possible toxicity.
The following neuromuscular agents are known or thought to interact with quinine causing respiratory difficulties :
pancuronium bromide, atracurium besylate, suxamethonium chloride, mivacurium chloride, pipecuronium bromide, rapacuronium bromide, alcuronium chloride, cisatracurium besylate, doxacurium chloride, gallamine triethiodide, metocurine iodide, decamethonium bromide or biiodatum, rocuronium bromide, vercuronium chloride and tubocurarine chloride.
Agents that enhance neuromuscular blocking drugs must be monitored if used concurrently with quinine. These drugs include :
• antiarrhythmics (lignocaine, procainamide, quinidine, nifedipine and verapamil), anticholinesterases (neostigmine and edrophonium),
• diuretics (frusemide and mannitol),
• antibacterials (some antibacterials in high concentrations may produce a muscle paralysis, which may be additive to or synergistic with that produced by neuromuscular blockers. This may be enhanced in patients with intracellular potassium deficiency or low plasma calcium concentration following large doses or renal impairment. The agents most commonly implicated are aminoglycosides, lincosamides, polymixins, vancomycin and rarely, tetracyclines. These should be used with care with quinine or monitored very closely.
• Ganglion blockers, including trimetaphan.
• General anaesthetics (Dose dependent enhancement by inhalation anaesthetics especially with competitive blockers. The greatest potentiation is from isoflurane, enflurane, desflurane and sevoflurane followed by halothane and cyclopropane.)
• Magnesium salts if given parenterally.
• Sympathomimetics (IV salbutamol has been reported to enhance the blockade obtained with pancuronium and vercuronium).
• Others, including antimyasthenics, haemolytics, neurotoxic medication, mefloquine, lithium and quinidine.
The use of quinine in combination with mefloquine is not recommended due to an increased risk of cardiac arrhythmias. Quinine and chloroquine may be antagonistic when used together in falciparum malaria.
SIDE EFFECTS :
Cinchonism :
When quinine is given repeatedly, a group of symptoms known as cinchonism occurs. Cinchonism symptoms include tinnitus, impaired hearing, headache, nausea, disturbed vision, vomiting, abdominal pain, diarrhoea and vertigo.
Haematological effects :
Haematological effects include acute haemolysis, thrombocytopenic purpura (which may be fatal), agranulocytosis and hypoprothrombinaemia.
CNS effects :
CNS effects include visual disturbances, blurred vision with scotomata, photophobia, diplopia, mydriasis, constricted visual fields, night-blindness and disturbed colour perception. In severe cases optic atrophy may result in blindness. Tinnitus, vertigo, deafness, headache, confusion and syncope.
Dermatological effects :
Dermatological effects include rashes, urticaria, pruritis, flushing of the skin, facial oedema and photosensitivity.
Asthma :
Treatment may precipitate asthma.
Cardiac effects :
Disturbances in cardiac rhythm on conduction, widening of the QRS complex, hypotension, ventricular tachycardia, angio-oedema, and angina symptoms in sensitive patients. Severe or even fatal cardiotoxicity can result from rapid IV administration if quinine.
Gastrointestinal effects :
Gastrointestinal affects include nausea, vomiting and epigastric pain.
Myasthenia effects :
Treatment may aggravate myasthenia gravis.
Other effects :
Other adverse effects may include hepatotoxicity, anuria, uraemia haemoglobinuria (rare) and aggravation of hypoglycaemia.
OVERDOSAGE :
Cinchonism may occur with therapeutic doses of quinine and symptoms include nausea, vomiting, tinnitus, deafness, headache, vasodilatation, and slightly disturbed vision. These symptoms may also occur in acute overdosage, but the visual disorders may be severe and there may be CNS disturbances and cardiotoxicity. A lethal dose or lethal plasma-quinine concentration has not been established but fatalities have been reported in adults after doses of 2 to 8 g and in children after 1 g. An analysis of 165 cases of acute quinine poisoning revealed that : 21 % had no symptoms, nausea with or without vomiting occurred in 47 %, visual disturbances in 42 %, tinnitus in 38 %, other auditory disturbances in 23 %, sinus tachycardia in 23%, and other ECG abnormalities in 8 %. Mild impairment of consciousness was reported in 14 % of the patients while 7 (4 %) patients had deeper grades of coma. Of the 5 patients who died, 4 developed intractable ventricular arrhythmias and the fifth had a Jacksonian fit followed by cardiac arrest.
The effects of oculotoxicity may include blurred vision, defective colour perception, visual field constriction, and total blindness. The onset of symptoms may vary from a few hours to a day or more after ingestion. Suggested mechanisms for the oculotoxicity of quinine include an action on the retinal vasculature to produce ischaemia or a direct toxic effect on the retina. Visual loss in one group of patients was associated with plasma-quinine concentrations in excess of 10 micrograms/ml. However, in another group plasma-quinine concentrations were considered to be an imprecise guide to predicting visual disturbances. The speed and degree of visual recovery varies. Of 70 patients with visual disturbances following quinine poisoning, 39 subsequently complained of a period
of total blindness. Permanent visual deficits remained in 19 of these but no patient had permanent bilateral blindness. All of the 31 patients who had had blurred vision recovered full visual acuity.
It is considered that the actions of quinine on the myocardium are similar to those of quinidine but that it is less potent. Sinus tachycardia and minor ECG changes are the most common cardiovascular effects. Conduction abnormalities and ventricular dysrhythmias may occur with severe poisoning. Ventricular tachycardia is mostly associated with cardiogenic shock or circulatory collapse. Hypokalaemia may also occur.
TREATMENT OF OVERDOSAGE :
Treatment is mostly symptomatic with attention being given to maintaining blood pressure, respiration, and renal function, and to treating arrhythmias. Vasodilators and stellate ganglion block have been used to prevent or reverse visual impairment but there is little evidence to support their use. In a study involving 16 patients with quinine poisoning forced acid diuresis, haemodialysis, haemoperfusion, or plasma exchange were all found to be ineffective in increasing quinine elimination. Stellate ganglion block has been recommended to prevent or reverse retinal damage, the rationale being that quinine-induced oculotoxicity might arise from retinal arteriolar constriction. However, clinical studies have failed to find sufficient improvement to justify its use. There has been a report of the intravenous administration of nitrates producing beneficial responses in 2 patients.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 Months.
PRESENTATION :
Quinine Dihydrochloride Sterile Concentrate B.P. is supplied as per below table:

Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular