100 mg, 200 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
DANAZOL CAPSULES USP (Danazol) is gonadotropin inhibitor. Chemically, danazol is Pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol, (17α)-. The molecular formula is C22H27NO2 and molecular weight is 337.46.
STRUCTURAL FORMULA :
Its structural formula is :
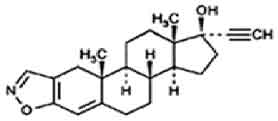
DANAZOL CAPSULES USP are hard gelatin capsules containing white to pale yellow crystalline powder.
COMPOSITION :
Each hard gelatin capsule contains :
Danazol USP 200 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Danazol suppresses the pituitary-ovarian axis possibly by inhibiting the output of pituitary gonadotropins. Danazol also depresses the preovulatory surge in output of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and therefore reduces ovarian oestrogen production. Danazol also may directly inhibit ovarian steroidogenesis, bind to androgen, progesterone, and glucocorticoid receptors, bind to sex-hormone-binding globulin and corticosteroid-binding globulin, and increase the metabolic clearance rate of progesterone.
Endometriosis :
As a consequence of suppression of ovarian function, both normal and ectopic endometrial tissues become inactive and atrophic. As a result, anovulation and associate amenorrhoea occur.
Fibrocystic breast disease :
The exact mechanism of action is unknown, but may be related to suppressed oestrogenic stimulation as a result of decreased ovarian production of oestrogen. A direct effect on steroid receptor sites in breast tissue also is possible. Disappearance of nodularity, relief of pain and tenderness, and possibly changes in the menstrual pattern result.
Hereditary angioedema : Danazol corrects the underlying biochemical deficiency by increasing serum concentrations of the deficient C1 esterase inhibitor, resulting serum concentration of the C4 component of the complement system.
PHARMACOKINETICS :
Following oral administration and absorption, danazol is extensively metabolised. However, plasma levels of unchanged danazol rise quickly, indicating a rapid onset of absorption. Peak plasma levels have been recorded occurring within 2-8 hours of medicine administration in a number of studies. A considerable difference in peak plasma levels has been observed in individuals receiving the same dosage, and in bioavailability studies, levels do not increase in proportion to the administered dose. When the dose of danazol is doubled the increase in plasma levels is about 35 % to 40 %. The half-life of danazol has been estimated as 4.5, 6, 14.7 and 29 hours; wide differences occur among individual subjects. Tissue distribution studies have demonstrated the continued presence of radioactivity in the intestines and stomach suggesting that danazol and its metabolites may undergo enterohepatic circulation. No consistent localisation of radioactivity has been found in any tissue other than the adrenal gland and the organs of excretion. Danazol has no significant effect on prolactin levels, or on thyroid or adrenal function. Reduced serum thyroxine levels may occur and are attributed to competition between thyroxine and danazol for binding sites on thyroxine-binding plasma proteins. In humans the major urinary metabolites of danazol are 2-hydroxymethylethisterone and ethisterone. Other minor urinary metabolites identified are Δ-2 2hydroxymethylethisterone, 6β-hydroxy-2-hydroxymethylethisterone and Δ-6β-hydroxy-2-hydroxymethylethisterone. None of these metabolites have been found to exhibit antigonadotrophic activity.
INDICATIONS :
Endometriosis :
Danazol is indicated for use in the treatment of endometriosis amenable to hormonal management.
Menorrhagia :
Danazol is indicated for the short-term (up to 6 months) management of primary menorrhagia.
Fibrocystic Disease of the Breast :
Danazol is indicated for the treatment of fibrocystic disease of the breast in both pre- and post-menopausal women.
Hereditary Angioedema :
Danazol is indicated for the prophylaxis of attacks of hereditary angioedema of a severe or life-threatening nature, in male and female patients.
Administration :
DANAZOL CAPSULES USP is for oral administration.
Dosage :
Therapy in female patients should begin during menstruation, otherwise appropriate tests should be performed to ensure the patient is not pregnant. A non-hormonal method of contraception should be recommended.
Endometriosis :
200 - 800 mg DANAZOL CAPSULES USP daily in two to four divided doses. It is recommended that treatment be initiated with a dosage of 800 mg daily in 4 divided doses. In some patients, it may be possible to maintain improvement with a reduced dosage once a satisfactory response has been obtained. Treatment should continue uninterrupted for 3 to 6 months, but may be extended to 9 months if necessary. Shorter courses have been used as adjuncts to surgery.
Menorrhagia :
A course of 200 - 400 mg DANAZOL CAPSULES USP daily in divided doses for up to 6 months. 200 mg is usually sufficient to reduce menstrual blood flow to acceptable limits.
Fibrocystic Disease of the Breast :
100 - 400 mg DANAZOL CAPSULES USP daily in divided doses. Dosage should be adjusted according to symptom intensity.
Hereditary Angioedema :
200 - 600 mg DANAZOL CAPSULES USP daily in divided doses. Dosage should be kept as low as possible with adjustment to meet individual patient requirements. Consideration should be given to interrupting treatment after an attack-free period.
CONTRAINDICATIONS :
- Pregnancy.
- Breast feeding.
- Markedly impaired hepatic, renal or cardiac function.
- Porphyria.
- Active thrombosis or thromboembolic disease and a history of such events.
- Androgen dependent tumour.
- Undiagnosed abnormal genital bleeding.
WARNINGS :
In the event of virilisation, DANAZOL CAPSULES USP should be withdrawn. Androgenic reactions generally prove reversible, but continued use of DANAZOL CAPSULES USP after evidence of androgenic virilisation increases the risk of irreversible androgenic effects.
DANAZOL CAPSULES USP should be stopped if any clinically significant adverse event arises, and particularly if there is evidence of papilloedema, headache, visual disturbances or other signs or symptoms of raised intracranial pressure, jaundice or other indication of significant hepatic disturbance, thrombosis or thromboembolism. Whilst a course of therapy may need to be repeated, care should be observed as no safety data are available in relation to repeated courses of treatment over time. The long-term risk of 17-alkylated steroids (including benign hepatic adenomata, peliosis hepatis and hepatic carcinoma), should be considered when danazol, which is chemically related to those compounds, is used.
Data, from two case-control epidemiological studies, were pooled to examine the relationship between endometriosis, endometriosis treatments and ovarian cancer. These preliminary results suggest that the use of danazol might increase the baseline risk of ovarian cancer in - patients treated for endometriosis. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
PRECAUTIONS :
In view of its pharmacology, known interactions and side effects, particular care should be observed when using DANAZOL CAPSULES USP in patients with hepatic or renal disease, hypertension or other cardiovascular disease and in any state which may be exacerbated by fluid retention as well as in diabetes mellitus, polycythaemia, epilepsy, lipoprotein disorder, and in those who have shown marked or persistent androgenic reaction to previous gonadal steroid therapy. Caution is advised in patients with migraine. Until more is known, caution is advised in the use of DANAZOL CAPSULES USP in the presence of known or suspected malignant disease. Before treatment initiation, the presence of hormone-dependent carcinoma should be excluded at least by careful clinical examination, as well as if breast nodules persist or enlarge during danazol treatment. In addition to clinical monitoring in all patients, appropriate laboratory monitoring should be considered which may include periodic measurement of hepatic function and haematological state. For long-term treatment (> 6 months) or repeated courses of treatment, biannual hepatic ultrasonography is recommended. Danazol should be initiated during menstruation. An effective, non-hormonal method of contraception should be employed.
The lowest effective dose of DANAZOL CAPSULES USP should always be sought.
Pregnancy : Category X
If taken by the mother at or after 8 weeks post conception, danazol may cause virilisation of the female foetus. Prior to 8 weeks it has no virilising effects. Danazol may not inhibit ovulation in all women. Therefore it is essential that barrier methods of contraception are used during danazol treatment. Anabolic steroids and other substances may have a virilising effect on the female foetus. Therefore the use of danazol in pregnancy is contraindicated. Pregnancy should be excluded before commencing therapy and therapy should commence during menstruation.
A non-hormonal method of contraception should be recommended. If a patient becomes pregnant while taking danazol, administration should be discontinued and the patient should be apprised of the potential risk of the foetus. If a patient suspects she has become pregnant during treatment, she should cease danazol treatment and consult her physician. Exposure to danazol in utero may result in androgenic effects on the female foetus; reports to date comprise clitoral hypertrophy, labial fusion, urogenital sinus defect, vaginal atresia and ambiguous genitalia.
Nursing mothers :
It is not known if danazol is excreted in breast milk and because of the potential for serious adverse reactions in nursing infants from danazol, a decision should be made whether to discontinue nursing or discontinue the danazol, taking into account the importance of the medication to the mother.
Paediatric Use :
Safety and efficacy in children have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Warfarin : Prolongation of prothrombin time occurs in patients stabilised on warfarin.
Anticonvulsants : Therapy with danazol may reduce the plasma clearance of carbamazepine, increasing its elimination half-life and plasma concentration and, may affect responsiveness to this agent and to phenytoin.
Cyclosporin and tacrolimus : Therapy with danazol may cause an increase in the serum levels of concomitantly administered cyclosporin and tacrolimus.
Oral Contraceptives : Although no specific interaction has been recorded, it is recommended that oral contraceptives should not be used concurrently with danazol.
Antidiabetic Therapy : Danazol can cause insulin resistance.
Concomitant Steroids : It is likely that interactions between danazol and gonadal steroid therapy would occur.
Antihypertensives : Danazol can diminish the effectiveness of antihypertensive agents.
Laboratory Tests : Danazol treatment may interfere with laboratory determinations of testosterone, androstenedione, and dehydroepiandrosterone or plasma proteins.
SIDE EFFECTS :
In general, the side effects associated with danazol therapy are attributable to its pharmacological activity; these effects reflect danazol’s weak androgenic and anabolic activity and/or the gonadal suppression which results from therapy.
Androgenic/Anabolic Effects :
Weight gain (4 %), acne (13 %) and seborrhoea (2 %). Mild hirsutism (5 %), oedema (6 %), hair loss, voice change (3 %), which may take the form of hoarseness, sore throat or of instability or deepening of the pitch, may occur and may persist after cessation of therapy. Hypertrophy of the clitoris and fluid retention are rare.
Endocrine Effects :
Menstrual disturbances in the form of spotting, alteration of the timing of the cycle and amenorrhoea. Although cyclical bleeding and ovulation usually return within 60 - 90 days after discontinuation of danazol, persistent amenorrhoea has occasionally been reported.
Flushing (6 %), sweating (3 %) and vaginal dryness and irritation (4 %) may reflect lowering of oestrogen. Changes in breast size are uncommon. Very rarely, abnormalities in semen volume, viscosity, sperm count, and motility may occur in males receiving long-term therapy. Testicular atrophy may occur rarely.
Hepatic Effects :
Hepatic dysfunction, as evidenced by elevated serum enzymes and/or jaundice have been reported in patients receiving a daily dosage of danazol of 400 mg or more. Serious hepatic toxicity including cholestatic jaundice, peliosis hepatitis, hepatic adenoma and malignant hepatic tumour have been reported.
Biochemical Abnormalities :
Alteration in values for laboratory tests may occur during danazol therapy including : CPK, glucose tolerance, glucagon, sex hormone binding globulin, other plasma proteins, raised SGOT, decreased PBI, blunted cyclical surges of LH and induction of aminolevulinic acid (ALA) synthetase. Other events include reduction in thyroid binding globulin and T4, with increased uptake of T3 but without disturbance of thyroid stimulating hormone or of free thyroxine index. Total cholesterol and low-density lipoprotein cholesterol may increase and high-density lipoprotein cholesterol may decrease. A decrease in apolipoproteins AI and AII has been reported.
The following reactions have also been reported :
Allergic : Uncommonly urticaria, pruritis and rarely nasal congestion.
Skin : Rashes (3 %) (maculopapular, vesicular, papular, purpuric, petechial), sometimes associated with facial oedema, fever or sun sensitivity. Very rarely Stevens-Johnson syndrome, inflammatory erythematous nodules, erythema multiforme, and skin pigmentation.
Gastrointestinal : Nausea (2 %), vomiting, constipation, indigestion, gastroenteritis and rarely pancreatitis.
Genitourinary : Very rarely haematuria.
Musculoskeletal : Muscle cramps or spasms, or pains, muscle tremors, fasiculation, arthralgia, joint lock-up, joint swelling, pain in back, neck or extremities and rarely carpal tunnel syndrome which may be secondary to fluid retention.
CNS : Commonly headache, emotional lability, irritability, nervousness, anxiety, changes in appetite and depression. Rarely weakness, faintness, dizziness, vertigo, fatigue, tremor and benign intracranial hypertension. Very rarely provocation of migraine and aggravation of epilepsy. Paraesthesias, sleep disorders, chills, cataracts and rarely Guillian-Barre syndrome have also been reported.
Haematologic : Increased red cell and platelet count; leucocytosis, polycythemia, leucopenia and thrombocytopenia. Very rarely reversible erythrocytosis, eosinophilia and splenic peliosis have been noted.
Cardiovascular : Rarely elevation in blood pressure and exacerbation of existing hypertension, palpitation and tachycardia. Thrombotic events have also been observed, including sagittal sinus and cerebrovascular thrombosis as well as arterial thrombosis. Cases of myocardial infarction have been reported.
Ophthalmic : Rarely visual disturbances such as blurring of vision and difficulty in focusing, difficulty in wearing contact lenses and refraction disorders requiring correction.
Other : Increased insulin requirements in diabetic patients, changes in libido, pelvic pain, epigastric pain, interstitial pneumonitis, pleuritic pain, bleeding gums, fever, Bartholin’s cyst and rarely nipple discharge.
OVERDOSAGE :
Available evidence suggests that acute overdosage would be unlikely to give rise to immediate serious reaction. In the case of acute overdose consideration should be given to reducing the absorption of the drug with activated charcoal and the patient should be kept under observation in case of any delayed reactions.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DANAZOL CAPSULES USP contains danazol USP 200 mg
3 blisters of 10 capsules per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular