40 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
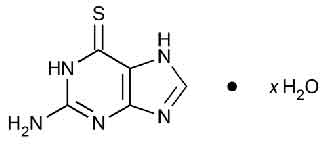
THIOGUANINE TABLETS USP (Thioguanine) belongs to a group of medicines called anti-neoplastic agents. Within this group, THIOGUANINE TABLETS USP belongs to a class of medicines called purine antimetabolites. Chemically, Thioguanine is 6 H-purine-6-thione, 2-amino-1,7-dihydro. The molecular formula is C5H5N5S · x H2O (anhydrous) and molecular weight is 167.19.
STRUCTURAL FORMULA :
Its structural formula is :
THIOGUANINE TABLETS USP is white to yellowish white coloured, circular, uncoated scored tablet.
COMPOSITION :
Each uncoated tablet contains :
Thioguanine USP 40 mg
Excipients q.s.
ACTIONS :
Thioguanine is a sulphydryl analogue of guanine and behaves as a purine antimetabolite. It is activated to its nucleotide, thioguanylic acid. Thioguanine metabolites inhibit de novo purine synthesis and purine nucleotide interconversions. Thioguanine is also incorporated into nucleic acids and DNA (deoxyribonucleic acid) incorporation is claimed to contribute to the agents cytotoxicity. Cross resistance usually exists between thioguanine and mercaptopurine, and it is not to be expected that patients resistant to one will respond to the other.
PHARMACOKINETICS :
Thioguanine is incompletely and variably absorbed from the gastrointestinal tract; on average about 30 % of a dose is absorbed after oral doses. It is rapidly activated in the body by intracellular conversion to its nucleotide, thioguanylic acid and its thioguanosine phosphate derivatives. With repeated doses increasing amounts of the nucleotide are incorporated into DNA. Very little unchanged thioguanine has been detected circulating in the blood but the half-life of the nucleotide in the tissues is prolonged. Thioguanine is inactivated primarily by methylation to aminomethylthiopurine; small amounts are deaminated to thioxanthine, and may go on to be oxidised by xanthine oxidase to thiouric acid, but inactivation is essentially independent of xanthine oxidase and is not affected by inhibition of the enzyme. It is excreted in the urine almost entirely as metabolites; only negligible amounts of thioguanine have been detected. Thioguanine does not appear to cross the blood-brain barrier to a significant extent; very little is found in CSF after normal clinical doses. It crosses the placenta.
INDICATIONS :
Thioguanine is an analogue of the naturally occurring purine, guanine, and is an antineoplastic with actions and uses similar to those of mercaptopurine. It appears to cause fewer gastrointestinal reactions but cross-resistance exists so that patients who do not respond to one are unlikely to respond to the other. Thioguanine may be given by mouth, usually with cytarabine and an anthracycline, in the induction and maintenance of remissions in acute myeloid leukaemia, but there is some uncertainty about its value. It has also been used in other malignancies including acute lymphoblastic leukaemia and chronic myeloid leukaemia.
Administration :
For oral use.
Dosage :
Doses up to 200 mg/m2 daily have been given for 5 to 20 days as part of a combined induction regimen; similar doses have been used in children as well as lower doses of 60 to 75 mg/m2. For maintenance therapy, intermittent or continuous daily doses of up to 200 mg/m2 have been used in both adults and children. A dose of 2 mg/kg daily increased after 4 weeks, if there is no response or toxicity allows, to 3 mg/kg daily may be given to adults and children in those rare cases when single agent therapy is considered appropriate.
Blood counts should be made frequently, particularly during induction and when thioguanine is given with other antineoplastic agents. Therapy should be withdrawn at the first sign of severe bone-marrow depression.
CONTRAINDICATIONS :
Thioguanine should not be used in patients whose disease has demonstrated prior resistance to this drug. In animals and humans, there is usually complete cross-resistance between Mercaptopurine and Thioguanine. THIOGUANINE TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
SINCE DRUGS USED IN CANCER CHEMOTHERAPY ARE POTENTIALLY HAZARDOUS, IT IS RECOMMENDED THAT ONLY PHYSICIANS EXPERIENCED WITH THE RISKS OF THIOGUANINE AND KNOWLEDGEABLE IN THE NATURAL HISTORY OF ACUTE NONLYMPHOCYTIC LEUKAEMIAS ADMINISTER THIS DRUG.
The most consistent, dose-related toxicity is bone marrow suppression. This may be manifested by anaemia, leucopenia, thrombocytopaenia, or any combination of these. Any one of these findings may also reflect progression of the underlying disease. Since thioguanine may have a delayed effect, it is important to withdraw the medication temporarily at the first sign of an abnormally large fall in any of the formed elements of the blood. There are individuals with an inherited deficiency of the enzyme thiopurine methyltransferase (TPMT) who may be unusually sensitive to the myelosuppressive effects of thioguanine and prone to developing rapid bone marrow suppression following the initiation of treatment. Substantial dosage reductions may be required to avoid the development of life-threatening bone marrow suppression in these patients. Prescribers should be aware that some laboratories offer testing for TPMT deficiency. Since bone marrow suppression may be associated with factors other than TPMT deficiency, TPMT testing may not identify all patients at risk for severe toxicity. Therefore, close monitoring of clinical and hematologic parameters is important. Bone marrow suppression could be exacerbated by coadministration with drugs that inhibit TPMT, such as olsalazine, mesalazine, or sulphasalazine.
PRECAUTIONS :
General :
Although the primary toxicity of thioguanine is myelosuppression, other toxicities have occasionally been observed, particularly when thioguanine is used in combination with other cancer chemotherapeutic agents. A few cases of jaundice have been reported in patients with leukaemia receiving thioguanine. Among these were 2 adult male patients and 4 pediatric patients with acute myelogenous leukaemia and an adult male with acute lymphocytic leukaemia who developed veno-occlusive hepatic disease while receiving chemotherapy for their leukaemia. Six patients had received cytarabine prior to treatment with thioguanine, and some were receiving other chemotherapy in addition to thioguanine when they became symptomatic. While veno-occlusive hepatic disease has not been reported in patients treated with thioguanine alone, it is recommended that thioguanine be withheld if there is evidence of toxic hepatitis or biliary stasis, and that appropriate clinical and laboratory investigations be initiated to establish the etiology of the hepatic dysfunction. Deterioration in liver function studies during thioguanine therapy should prompt discontinuation of treatment and a search for an explanation of the hepatotoxicity. THIOGUANINE TABLETS USP should be used cautiously in diabetic patients.
Laboratory Tests :
Prescribers should be aware that some laboratories offer testing for thiopurine methyltransferase (TPMT) deficiency. It is advisable to monitor liver function tests (serum transaminases, alkaline phosphatase, bilirubin) at weekly intervals when first beginning therapy and at monthly intervals thereafter. It may be advisable to perform liver function tests more frequently in patients with known pre-existing liver disease or in patients who are receiving thioguanine and other hepatotoxic drugs. Patients should be instructed to discontinue thioguanine immediately if clinical jaundice is detected.
Pregnancy : Pregnancy Category D
THIOGUANINE TABLETS USP like other cytotoxic agents is potentially teratogenic. There have been isolated cases where men, who have received combinations of cytotoxic agents including THIOGUANINE TABLETS USP, have fathered children with congenital abnormalities. Its use should be avoided whenever possible during pregnancy, particularly during the first trimester. In any individual case the potential hazard to the foetus must be balanced against the expected benefit to the mother. As with all cytotoxic chemotherapy, adequate contraceptive precautions should be advised when either partner is receiving THIOGUANINE TABLETS USP.
Nursing mothers :
There are no reports documenting the presence of THIOGUANINE TABLETS USP or its metabolites in maternal milk. It is suggested that mothers receiving THIOGUANINE TABLETS USP should not breast feed.
CARCINOGENESIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY :
In view of its action on cellular DNA, thioguanine is potentially mutagenic and carcinogenic, and consideration should be given to the theoretical risk of carcinogenesis when thioguanine is administered.
INTERACTIONS AND INCOMPATIBILITIES :
Vaccinations with live organism vaccines are not recommended in immunocompromised individuals. There is usually complete cross-resistance between mercaptopurine and Thioguanine. As there is in vitro evidence that aminosalicylate derivatives (e.g., olsalazine, mesalazine, or sulphasalazine) inhibit the TPMT enzyme, they should be administered with caution to patients receiving concurrent thioguanine therapy.
SIDE EFFECTS :
The most frequent adverse reaction to thioguanine is myelosuppression. The induction of complete remission of acute myelogenous leukaemia usually requires combination chemotherapy in dosages which produce marrow hypoplasia. Since consolidation and maintenance of remission are also effected by multiple-drug regimens whose component agents cause myelosuppression, pancytopenia is observed in nearly all patients. Dosages and schedules must be adjusted to prevent life-threatening cytopenias whenever these adverse reactions are observed. Hyperuricemia frequently occurs in patients receiving thioguanine as a consequence of rapid cell lysis accompanying the antineoplastic effect. Adverse effects can be minimized by increased hydration, urine alkalinization, and the prophylactic administration of a xanthine oxidase inhibitor such as allopurinol. Unlike mercaptopurine and azathioprine, thioguanine may be continued in the usual dosage when allopurinol is used conjointly to inhibit uric acid formation. Less frequent adverse reactions include nausea, vomiting, anorexia, and stomatitis. Intestinal necrosis and perforation have been reported in patients who received multiple-drug chemotherapy including thioguanine.
Hepatic Effects : Liver enzyme and other liver function studies are occasionally abnormal. If jaundice, hepatomegaly, or anorexia with tenderness in the right hypochondrium occurs, thioguanine should be withheld until the exact etiology can be determined. There have been reports of veno-occlusive liver disease occurring in patients who received combination chemotherapy including thioguanine. Oesophageal varices have been reported in patients receiving continuous busulfan and thioguanine therapy for treatment of chronic myelogenous leukaemia.
OVERDOSAGE :
Signs and symptoms of overdosage may be immediate, such as nausea, vomiting, malaise, hypotension, and diaphoresis; or delayed, such as myelosuppression and azotemia. It is not known whether thioguanine is dialyzable. Haemodialysis is thought to be of marginal use due to the rapid intracellular incorporation of thioguanine into active metabolites with long persistence. The oral LD50 of thioguanine was determined to be 823 mg/kg ± 50.73 mg/kg and 740 mg/kg ± 45.24 mg/kg for male and female rats, respectively. Symptoms of overdosage may occur after a single dose of as little as 2.0 to 3.0 mg/kg thioguanine. As much as 35 mg/kg has been given in a single oral dose with reversible myelosuppression observed.
TREATMENT OF OVERDOSAGE :
There is no known pharmacologic antagonist of thioguanine. The drug should be discontinued immediately if unintended toxicity occurs during treatment. Severe hematologic toxicity may require supportive therapy with platelet transfusions for bleeding, and granulocyte transfusions and antibiotics if sepsis is documented. If a patient is seen immediately following an accidental overdosage of the drug, it may be useful to induce emesis.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
THIOGUANINE TABLETS USP contains Thioguanine USP 40 mg.
One bottle of 25 tablets per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular