2 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
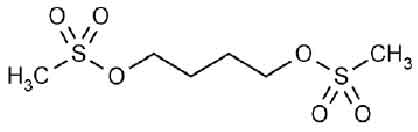
BUSULFAN TABLETS USP (Busulfan) is an antineoplastic agent. Chemically, Busulfan is 1,4-Butanediol, dimethanesulfonate The molecular formula is C6H14O6S2 and molecular weight is 246.30
STRUCTURAL FORMULA :
Its structural formula is :
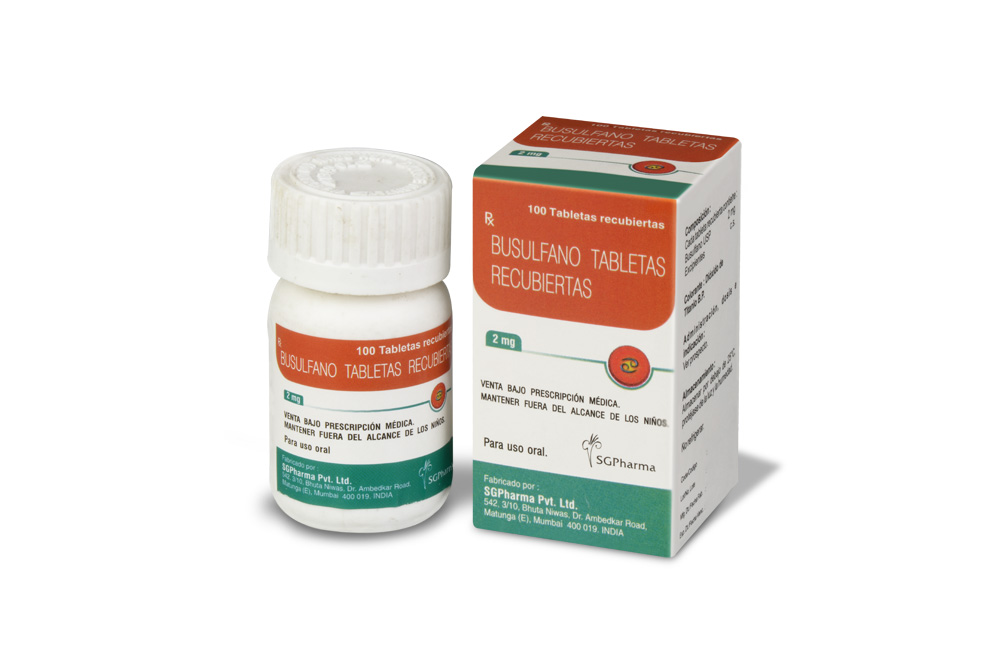
BUSULFAN TABLETS USP are white, biconvex, round film-coated tablets.
COMPOSITION :
Each film-coated tablet contains :
Busulfan USP 2 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTION :
Busulfan is an alkylating agent and reacts with N-7 position of guanine and interferes with DNA replication and transcription of RNA. It interferes with the normal function of DNA alkylation and cross linking the strands of DNA, which is responsible for its cytotoxic action. It has a more marked effect on myeloid cells (and it therefore, useful in treatment of CML) than on lymphoid cells. Busulfan is also very toxic to the haematopoietic stem cells and hence it is used at high dose in preparative regimens for BMT. Busulfan exhibits little immunosuppressive activity. It is a cell cycle non specific agent.
PHARMACOKINETICS :
Busulfan is well absorbed following oral administration. There is a lag period of 0.6 to 2 hr prior to detection in blood. The plasma elimination half-life is 2.5 hr and is similar for CSF. The drug appears to be extensively metabolized and renally excreted with little unchanged drug (≈ 30 %, injection) found in the urine. The clearance of busulfan is more rapid in children than in adults.
INDICATIONS :
Palliative treatment of chronic myelogenous leukaemia (oral); allogeneic bone marrow transplantation for chronic myelogenous leukaemia (IV).
Paediatric :
Palliative treatment of chronic myelogenous leukaemia (oral).Severe thrombocytosis, polycythemia vera, myelofibrosis ; bone marrow transplantation (oral).
Administration :
BUSULFAN TABLET USP are for oral administration.
Dosage :
The usual adult dose range for remission induction is 4 to 8 mg, total dose, daily. Dosing on a weight basis is the same for both paediatric patients and adults, approximately 60 mcg/kg of body weight or 1.8 mg/m2 of body surface, daily. Since the rate of fall of the leukocyte count is dose related, daily doses exceeding 4 mg per day should be reserved for patients with the most compelling symptoms; the greater the total daily dose, the greater is the possibility of inducing bone marrow aplasia. A decrease in the leukocyte count is not usually seen during the first 10 to 15 days of treatment; the leukocyte count may actually increase during this period and it should not be interpreted as resistance to the drug, nor should the dose be increased. Since the leukocyte count may continue to fall for more than 1 month after discontinuing the drug, it is important that busulfan be discontinued prior to the total leukocyte count falling into the normal range. When the total leukocyte count has declined to approximately 15,000/mcl, the drug should be withheld.
With a constant dose of busulfan, the total leukocyte count declines exponentially; a weekly plot of the leukocyte count on semi-logarithmic graph paper aids in predicting the time when therapy should be discontinued. With the recommended dose of busulfan, a normal leukocyte count is usually achieved in 12 to 20 weeks. During remission, the patient is examined at monthly intervals and treatment resumed with the induction dosage when the total leukocyte count reaches approximately 50,000/mcl. When remission is shorter than 3 months, maintenance therapy of 1 to 3 mg daily may be advisable in order to keep the hematological status under control and prevent rapid relapse.
CONTRAINDICATIONS :
Busulfan is contraindicated in patients in whom a definitive diagnosis of chronic myelogenous leukaemia has not been firmly established. Busulfan is contraindicated in patients who have previously suffered a hypersensitivity reaction to busulfan or any other component of the preparation. BUSULFAN TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
The most frequent, serious side effect of treatment with busulfan is the induction of bone marrow failure (which may or may not be anatomically hypoplastic) resulting in severe pancytopenia. The pancytopenia caused by busulfan may be more prolonged than that induced with other alkylating agents. It is generally felt that the usual cause of busulfan -induced pancytopenia is the failure to stop administration of the drug soon enough; individual idiosyncrasy to the drug does not seem to be an important factor. Busulfan should be used with extreme caution and exceptional vigilance in patients whose bone marrow reserve may have been compromised by prior irradiation or chemotherapy, or whose marrow function is recovering from previous cytotoxic therapy. Although recovery from busulfan -induced pancytopenia may take from 1 month to 2 years, this complication is potentially reversible, and the patient should be vigorously supported through any period of severe pancytopenia.
A rare, important complication of busulfan therapy is the development of bronchopulmonary dysplasia with pulmonary fibrosis. Symptoms have been reported to occur within 8 months to 10 years after initiation of therapy—the average duration of therapy being 4 years. The histologic findings associated with “busulfan lung” mimic those seen following pulmonary irradiation. Clinically, patients have reported the insidious onset of cough, dyspnea, and low-grade fever. In some cases, however, onset of symptoms may be acute. Pulmonary function studies have revealed diminished diffusion capacity and decreased pulmonary compliance. It is important to exclude more common conditions (such as opportunistic infections or leukaemic infiltration of the lungs) with appropriate diagnostic techniques. If measures such as sputum cultures, virologic studies, and exfoliative cytology fail to establish an etiology for the pulmonary infiltrates, lung biopsy may be necessary to establish the diagnosis. Treatment of established busulfan-induced pulmonary fibrosis is unsatisfactory; in most cases the patients have died within 6 months after the diagnosis was established. There is no specific therapy for this complication. Busulfan should be discontinued if this lung toxicity develops. The administration of corticosteroids has been suggested, but the results have not been impressive or uniformly successful. Busulfan may cause cellular dysplasia in many organs in addition to the lung. Cytologic abnormalities characterized by giant, hyperchromatic nuclei have been reported in lymph nodes, pancreas, thyroid, adrenal glands, liver, and bone marrow. This cytologic dysplasia may be severe enough to cause difficulty in interpretation of exfoliative cytologic examinations from the lung, bladder, breast, and the uterine cervix. In addition to the widespread epithelial dysplasia that has been observed during busulfan therapy, chromosome aberrations have been reported in cells from patients receiving busulfan.
Busulfan is mutagenic in mice and, possibly, in humans. Malignant tumors and acute leukaemia have been reported in patients who have received busulfan therapy, and this drug may be a human carcinogen.The World Health Organization has concluded that there is a causal relationship between Busulfan exposure and the development of secondary malignancies. Four cases of acute leukaemia occurred among 243 patients treated with busulfan as adjuvant chemotherapy following surgical resection of bronchogenic carcinoma. All 4 cases were from a subgroup of 19 of these 243 patients who developed pancytopenia while taking busulfan 5 to 8 years before leukaemia became clinically apparent. These findings suggest that busulfan is leukaemogenic, although its mode of action is uncertain.
Ovarian suppression and amenorrhoea with menopausal symptoms commonly occur during busulfan therapy in premenopausal patients. Busulfan has been associated with ovarian failure including failure to achieve puberty in females. Busulfan interferes with spermatogenesis in experimental animals, and there have been clinical reports of sterility, azoospermia, and testicular atrophy in male patient Hepatic veno-occlusive disease, which may be life threatening, has been reported in patients receiving busulfan, usually in combination with cyclophosphamide or other chemotherapeutic agents prior to bone marrow transplantation. Possible risk factors for the development of hepatic veno-occlusive disease include : total busulfan dose exceeding 16 mg/kg based on ideal body weight, and concurrent use of multiple alkylating agents. A clear cause-and-effect relationship with busulfan has not been demonstrated. Periodic measurement of serum transaminases, alkaline phosphatase, and bilirubin is indicated for early detection of hepatotoxicity. A reduced incidence of hepatic veno-occlusive disease and other regimen-related toxicities have been observed in patients treated with high-dose BUSULFAN TABLETS USP and cyclophosphamide when the first dose of cyclophosphamide has been delayed for > 24 hours after the last dose of busulfan. Cardiac tamponade has been reported in a small number of patients with thalassemia (2 % in one series) who received busulfan and cyclophosphamide as the preparatory regimen for bone marrow transplantation. In this series, the cardiac tamponade was often fatal. Abdominal pain and vomiting preceded the tamponade in most patients.
PRECAUTIONS :
General :
The most consistent, dose-related toxicity is bone marrow suppression. This may be manifest by anaemia, leucopoenia, thrombocytopenia, or any combination of these. It is imperative that patients be instructed to report promptly the development of fever, sore throat, signs of local infection, bleeding from any site, or symptoms suggestive of anaemia. Any one of these findings may indicate Busulfan toxicity; however, they may also indicate transformation of the disease to an acute “blastic” form. Since Busulfan may have a delayed effect, it is important to withdraw the medication temporarily at the first sign of an abnormally large or exceptionally rapid fall in any of the formed elements of the blood. Patients should never be allowed to take the drug without close medical supervision. Seizures have been reported in patients receiving busulfan. As with any potentially epileptogenic drug, caution should be exercised when administering busulfan to patients with a history of seizure disorder, head trauma, or receiving other potentially epileptogenic drugs. Some investigators have used prophylactic anticonvulsant therapy in this setting. BUSULFAN TABLETS USP should be used cautiously in diabetic patients.
Information for Patients :
Patients beginning therapy with busulfan should be informed of the importance of having periodic blood counts and to immediately report any unusual fever or bleeding. Aside from the major toxicity of myelosuppression, patients should be instructed to report any difficulty in breathing, persistent cough, or congestion. They should be told that diffuse pulmonary fibrosis is an infrequent, but serious and potentially life-threatening complication of long-term busulfan therapy. Patients should be alerted to report any signs of abrupt weakness, unusual fatigue, anorexia, weight loss, nausea and vomiting, and melanoderma that could be associated with a syndrome resembling adrenal insufficiency. Patients should never be allowed to take the drug without medical supervision and they should be informed that other encountered toxicities to busulfan include infertility, amenorrhoea, skin hyperpigmentation, drug hypersensitivity, dryness of the mucous membranes, and rarely, cataract formation. Women of childbearing potential should be advised to avoid becoming pregnant. The increased risk of a second malignancy should be explained to the patient.
Laboratory Tests :
It is recommended that evaluation of the haemoglobin or haematocrit, total white blood cell count and differential count, and quantitative platelet count be obtained weekly while the patient is on busulfan therapy. In cases where the cause of fluctuation in the formed elements of the peripheral blood is obscure, bone marrow examination may be useful for evaluation of marrow status. A decision to increase, decrease, continue, or discontinue a given dose of busulfan must be based not only on the absolute haematologic values, but also on the rapidity with which changes are occurring. The dosage of busulfan may need to be reduced if this agent is combined with other drugs whose primary toxicity is myelosuppression. Occasional patients may be unusually sensitive to busulfan administered at standard dosage and suffer neutropenia or thrombocytopenia after a relatively short exposure to the drug. Busulfan should not be used where facilities for complete blood counts, including quantitative platelet counts, are not available at weekly (or more frequent) intervals.
Drug Interactions :
Busulfan may cause additive myelosuppression when used with other myelosuppressive drugs. In one study, 12 of approximately 330 patients receiving continuous busulfan and thioguanine therapy for treatment of chronic myelogenous leukaemia were found to have portal hypertension and esophageal varices associated with abnormal liver function tests. Subsequent liver biopsies were performed in 4 of these patients, all of which showed evidence of nodular regenerative hyperplasia. Duration of combination therapy prior to the appearance of oesophageal varices ranged from 6 to 45 months. With the present analysis of the data, no cases of hepatotoxicity have appeared in the busulfan-alone arm of the study. Long-term continuous therapy with thioguanine and busulfan should be used with caution. Busulfan-induced pulmonary toxicity may be additive to the effects produced by other cytotoxic agents. The concomitant systemic administration of itraconazole to patients receiving high-dose busulfan may result in reduced busulfan clearance. Patients should be monitored for signs of busulfan toxicity when itraconazole is used concomitantly with busulfan.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The World Health Organization has concluded that there is a causal relationship between busulfan exposure and the development of secondary malignancies.
Pregnancy : Pregnancy Category D.
Nonteratogenic Effects : There have been reports in the literature of small infants being born after the mothers received busulfan during pregnancy, in particular, during the third trimester. One case was reported where an infant had mild anaemia and Neutrogena at birth after busulfan was administered to the mother from the eighth week of pregnancy to term.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because of the potential for tumorigenicity shown for busulfan in animal and human studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatrics :
Safety and efficacy have not been established.Clearance has been demonstrated to be greater in children than in adults, necessitating the development of alternative dosing regimens. Cardiac tamponade has been reported for patients with thalassaemia receiving high doses of oral busulfan and cyclophosphomides.
Geriatric Use :
Clinical studies of busulfan did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
INTERACTIONS AND INCOMPATIBILITIES :
Vaccinations with live organism vaccines are not recommended in immunocompromised individuals. The effects of other cytotoxics producing pulmonary toxicity may be additive. The administration of phenytoin to patients receiving high-dose BUSULFAN TABLETS USP may result in a decrease in the myeloblative effect. In patients receiving high-dose busulfan it has been reported that co-administration of itraconazole decreases clearance of busulfan by approximately 20 % with corresponding increases in plasma busulfan levels. Metronidazole has been reported to increase trough levels of busulfan by approximately 80 %. Fluconazole had no effect on Busulfan clearance. Consequently, high-dose busulfan in combination with itraconazole or metronidazole is reported to be associated with an increased risk of busulfan toxicity. A reduced incidence of hepatic veno-occlusive disease and other regimen-related toxicities have been observed in patients treated with high-dose BUSULFAN TABLETS USP and cyclophosphamide when the first dose of cyclophosphamide has been delayed for > 24 hours after the last dose of busulfan.
SIDE EFFECTS :
Haematological Effects :
The most frequent, serious, toxic effect of busulfan is dose-related myelosuppression resulting in leucopenia, thrombocytopenia, and anaemia. Myelosuppression is most frequently the result of a failure to discontinue dosage in the face of an undetected decrease in leukocyte or platelet counts. Aplastic anaemia (sometimes irreversible) has been reported rarely, often following long-term conventional doses and also high doses of busulfan.
Pulmonary :
Interstitial pulmonary fibrosis has been reported rarely, but it is a clinically significant adverse effect when observed and calls for immediate discontinuation of further administration of the drug. The role of corticosteroids in arresting or reversing the fibrosis has been reported to be beneficial in some cases and without effect in others.
Cardiac :
Cardiac tamponade has been reported in a small number of patients with thalassemia who received busulfan and cyclophosphamide as the preparatory regimen for bone marrow transplantation. One case of endocardial fibrosis has been reported in a 79-year-old woman who received a total dose of 7,200 mg of busulfan over a period of 9 years for the management of chronic myelogenous leukaemia. At autopsy, she was found to have endocardial fibrosis of the left ventricle in addition to interstitial pulmonary fibrosis.
Ocular :
Busulfan is capable of inducing cataracts in rats and there have been several reports indicating that this is a rare complication in humans.
Dermatologic :
Hyperpigmentation is the most common adverse skin reaction and occurs in 5 % to 10 % of patients, particularly those with a dark complexion.
Metabolic :
In a few cases, a clinical syndrome closely resembling adrenal insufficiency and characterized by weakness, severe fatigue, anorexia, weight loss, nausea and vomiting, and melanoderma has developed after prolonged busulfan therapy. The symptoms have sometimes been reversible when busulfan was withdrawn. Adrenal responsiveness to exogenously administered ACTH has usually been normal. However, pituitary function testing with metyrapone revealed a blunted urinary 17-hydroxycorticosteroid excretion in 2 patients. Following the discontinuation of busulfan (which was associated with clinical improvement), rechallenge with metyrapone revealed normal pituitary-adrenal function. Hyperuricaemia and/or hyperuricosuria are not uncommon in patients with chronic myelogenous leukemia. Additional rapid destruction of granulocytes may accompany the initiation of chemotherapy and increase the urate pool. Adverse effects can be minimized by increased hydration, urine alkalinization, and the prophylactic administration of a xanthine oxidase inhibitor such as allopurinol.
Hepatic Effects :
Esophageal varices have been reported in patients receiving continuous busulfan and thioguanine therapy for treatment of chronic myelogenous leukaemia. Hepatic veno-occlusive disease has been observed in patients receiving busulfan.
Miscellaneous :
Other reported adverse reactions include : urticaria, erythema multiforme, erythema nodosum, alopecia, porphyria cutanea tarda, excessive dryness and fragility of the skin with anhidrosis, dryness of the oral mucous membranes and cheilosis, gynaecomastia, cholestatic jaundice, and myasthenia gravis. Most of these are single case reports, and in many, a clear cause-and-effect relationship with busulfan has not been demonstrated. Seizures have been observed in patients receiving higher than recommended doses of busulfan
OVERDOSAGE :
Symptoms and signs :
The acute dose-limiting toxicity of BUSULFAN TABLETS USP in man is myelosuppression. The main effect of chronic overdose is bone marrow depression and pancytopenia.
TREATMENT OF OVERDOSAGE :
There is no known antidote to busulfan. Haemodialysis should be considered in the management of overdose as there is one report of successful haemodialysis of busulfan. Appropriate supportive treatment should be given during the period of haematological toxicity.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerator.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
BUSULFAN TABLETS USP contain busulfan USP 2 mg.
100 tablets are packed in a bottle.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular