160 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
SODIUM THIOSULFATE INJECTION (Sodium Thiosulfate) is an antidote (to cyanide poisoning) and an antineoplastic adjunct. Chemically, Sodium Thiosulfate is Thiosulfuric acid disodium salt, pentahydrate. The molecular formula is Na2S2O3,5H2O and molecular weight is 248.2.
STRUCTURAL FORMULA :
Its structural formula is :
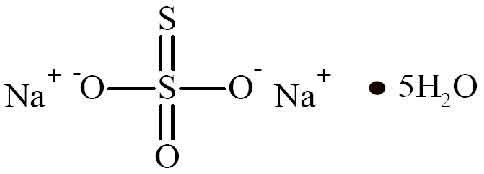
A sterile, clear, colourless solution filled in amber ampoule/vial of suitable size.
COMPOSITION :
1) Each ml contains :
Sodium Thiosulfate B.P. 80 mg
Water for Injections B.P. q.s.
2)Each ml contains :
Sodium Thiosulfate B.P. 100 mg
Water for Injections B.P. q.s.
3)Each ml contains :
Sodium Thiosulfate B.P. 250 mg
Water for Injections B.P. q.s.
4)Each ml contains :
Sodium Thiosulfate B.P. 500 mg
Water for Injections B.P. q.s.
ACTIONS :
Antidote (to cyanide poisoning) : Sodium thiosulfate acts as a sulfur donor for the endogenous sulfur transferase enzyme, rhodanese. Cyanide has a very high affinity for iron in the ferric state. It reacts readily with the trivalent (ferric) iron of mitochondrial cytochrome oxidase, thereby inhibiting cellular respiration, resulting in lactic acidosis and cytotoxic hypoxia. Sodium nitrite reacts with haemoglobin to form methaemoglobin, which competes with cytochrome oxidase for the cyanide ion. Cyanide preferentially binds to methaemoglobin to form cyanmethaemoglobin and restore the activity of cytochrome oxidase. As cyanide dissociates from methemoglobin, sodium thiosulfate facilitates its conversion by rhodanese to thiocyanate, a less toxic ion.
Antineoplastic adjunct :
The mechanism by which sodium thiosulfate reduces the nephrotoxicity caused by cisplatin is not fully understood. However, it is believed that sodium thiosulfate reacts covalently with cisplatin to form a non-toxic complex that is more efficiently eliminated than non-protein bound cisplatin. It is also believed that sodium thiosulfate protects against nephrotoxicity by reducing delivery of cisplatin to the kidneys and by neutralizing cisplatin in the kidneys where sodium thiosulfate is highly concentrated.
PHARMACOKINETICS :
Distribution : Distributed throughout the cellular fluid.
Half-life : Thiocyanate : 3 to 7 days. May be doubled or tripled in the presence of renal failure.
Thiosulfate : 15 to 20 minutes.
Elimination :
Renal : Antidote (to cyanide poisoning) : Primarily as thiocyanate. Antineoplastic adjunct : As a non-toxic sodium thiosulfate / cisplatin complex.
In dialysis : Dialysis is of no value in removing cyanide, but may be used to increase elimination of thiocyanate.
INDICATIONS :
Toxicity, Cyanide (treatment adjunct) :
SODIUM THIOSULFATE INJECTION, in conjunction with sodium nitrite, is indicated for use as an antidote in the treatment of cyanide poisoning.
Cyanide Toxicity, Sodium Nitroprusside-induced (prophylaxis) :
SODIUM THIOSULFATE INJECTION may be used to prevent cyanide toxicity caused by rapid infusion of sodium nitroprusside. Sodium nitroprusside infusion rates greater than 2 mcg per kg of body weight per minute generate cyanide ion faster than the body normally can eliminate it. The administration of SODIUM THIOSULFATE INJECTION greatly increases the body’s capacity for cyanide elimination.
Nephrotoxicity, Cisplatin-induced (prophylaxis) :
SODIUM THIOSULFATE INJECTION may be used with intraperitoneal cisplatin to reduce the toxicity associated with chemotherapy. SODIUM THIOSULFATE INJECTION, administered concurrently with cisplatin in the treatment of ovarian carcinoma, has been reported to reduce the dose-related nephrotoxicity of cisplatin, thereby allowing the dose of cisplatin to be increased.
Administration :
SODIUM THIOSULFATE INJECTION is administered by slow intravenous injection. If sodium nitrite is administered in the treatment of cyanide poisoning, sodium thiosulfate should be administered immediately following the sodium nitrite infusion.
The following procedure must be used for the compounding and administration of Sodium Thiosulfate Injection.
1.Perform a visual inspection on the ampoule/vial prior to withdrawal of the contents.
DO NOT USE IF PARTICULATES ARE PRESENT. USE A NEW AMPOULE/VIAL.
2.Use a 5 micron filter needle to withdraw the required calculated volume of Sodium Thiosulfate Injection.
NOTE : Sodium Thiosulfate Injection is to be administered intravenously by either the I.V. push method or after further dilution in a larger volume of I.V. fluid
3.If the I.V. push method is used, withdraw contents of Sodium Thiosulfate Injection ampoule/vial with a 5 micron filter needle. Replace filter needle with appropriate needle for injection before administering to patients.
4.If the I.V. infusion method is used, remove the filter needle and attach an appropriate needle to the syringe before adding to a larger volume of I.V. fluid and prior to patient administration. Visually inspect the final I.V. admixture solution.
DO NOT USE IF PARTICULATES ARE VISIBLE. DISCARD ADMIXTURE AND USE A NEW AMPOULE/VIAL.
5.Use a 0.22 micron in-line filter when administering the final I.V. admixture to patients.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
General Dosing Information :
For treatment of Cyanide toxicity :
Cyanide poisoning is rapidly fatal. Inhalation of cyanide gas produces symptoms of cyanide toxicity within seconds, followed by death within minutes. Oral ingestion of cyanide produces symptoms of toxicity within minutes, followed by death within minutes or hours. Blood cyanide concentrations often are not available for several hours. Therefore, therapy should be instituted immediately based upon reasonable suspicion of cyanide toxicity. Supportive therapy includes moving the patient to an uncontaminated area and/or removing contaminated clothing; administering 100 % oxygen; controlling seizures with anticonvulsants; correcting metabolic acidosis with bicarbonate; and supporting pulse and blood pressure with fluids, atropine, or vasopressors. These measures alone may allow survival in relatively mild cases of cyanide toxicity. In more severe cases of cyanide toxicity, chances of survival are increased by specific antidote administration. Antidotal therapy should be started by breaking an amyl nitrite inhalant, holding it in front of the patient’s mouth, and allowing the patient to inhale for 15 seconds. The inhalant should then be taken away for 15 seconds. This procedure may be repeated until an intravenous line is established and sodium nitrite is prepared. Amyl nitrite should not be administered if an intravenous line already has been established and the sodium nitrite is readily available. Amyl nitrite, if given, should then be discontinued and sodium nitrite administered intravenously in a dose of 300 mg (for adults) and 180 to 240 mg (6 to 8 ml) per square meter of body surface area (approximately 6 mg [0.2 ml] per kg of body weight) (for children), at a rate of 75 to 150 mg (2.5 to 5 ml) per minute. The paediatric dose should not exceed 300 mg (10 ml). Blood pressure and methaemoglobin concentrations should be monitored closely, and the administration of sodium nitrite discontinued if the systolic blood pressure goes below 80 mm Hg. The methaemoglobin concentration should not exceed 40 % in adults or 30 % in children. Sodium thiosulfate should be administered intravenously, immediately following the infusion of sodium nitrite. If signs of cyanide toxicity are still present 2 hours following administration of sodium nitrite and sodium thiosulfate, administration of both may be repeated at one-half the original dose.
Usual adult and adolescent dose :
Cyanide toxicity : Intravenous, 12.5 gm (50 ml of a 25 % solution) administered at a rate of 0.625 to 1.25 gm/minute (2.5 to 5 ml/minute).
Cyanide toxicity, Sodium Nitroprusside-induced : Intravenous, administered concurrently with sodium nitroprusside at 5 to 10 times the rate of sodium nitroprusside.
Nephrotoxicity, Cisplatin-induced : Intravenous, no standard dosing regimen has been established; however, experts recommend an initial loading dose of 4 gm/square meter of body surface area administered just before administration of cisplatin, followed by an intravenous infusion of 12 gm/square meter of body surface area administered over six hours beginning at the same time as cisplatin instillation.
WARNINGS AND PRECAUTIONS :
SODIUM THIOSULFATE INJECTION should be administered with caution in patients sensitive to sodium thiosulfate.
SODIUM THIOSULFATE INJECTION should also be administered with caution in patients with hypertension, since sodium thiosulfate may exacerbate the condition.
SODIUM THIOSULFATE INJECTION should be administered with caution in patients with oedematous sodium retaining conditions, such as cirrhosis of the liver, congestive heart failure, renal function impairment, and toxaemia of pregnancy, since sodium thiosulfate may also exacerbate these conditions. Sodium thiosulfate drug product may contain trace impurities of sodium sulfite. The presence of a trace amount of sulfites in this product should not deter administration of the drug for treatment of emergency situations, even if the patient is sulfite-sensitive.
Pregnancy :
Teratogenic Effects. Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Sodium Thiosulfate Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus. There are no reported epidemiological studies of congenital anomalies in infants born to women treated with sodium thiosulfate during pregnancy. In animal studies, there are no teratogenic effects in offspring of hamsters treated during pregnancy with sodium thiosulfate in doses similar to those given intravenously to treat cyanide poisoning in humans. Other studies suggest that treatment with sodium thiosulfate ameliorates the teratogenic effects of maternal cyanide poisoning in hamsters. In other studies, sodium thiosulfate was not embryotoxic or teratogenic in mice, rats, hamsters, or rabbits at maternal doses of up to 550, 400, 400 and 580 mg/kg/day, respectively.
Nursing mothers :
It is not known whether sodium thiosulfate is excreted in human milk. Because Sodium Thiosulfate Injection may be administered in life-threatening situations, breast-feeding is not a contraindication to its use. Because many drugs are excreted in human milk, caution should be exercised following Sodium Thiosulfate Injection administration to a nursing woman. There are no data to determine when breastfeeding may be safely restarted following administration of sodium thiosulfate.
Paediatric Use :
There are case reports in the medical literature of sodium nitrite in conjunction with sodium thiosulfate being administered to paediatric patients with cyanide poisoning; however, there have been no clinical studies to evaluate the safety or efficacy of sodium thiosulfate in the paediatric population. As for adult patients, dosing recommendations for paediatric patients have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
Geriatric Use :
Sodium thiosulfate is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Renal Disease :
Sodium thiosulfate is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
INTERACTIONS AND INCOMPATIBILITIES :
Chemical incompatibility has been reported between SODIUM THIOSULFATE INJECTION and hydroxocobalamin and these drugs should not be administered simultaneously through the same I.V. line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same I.V. line.
SIDE EFFECTS :
Sodium thiosulfate has low toxicity, and adverse reactions at the recommended doses are usually mild.
Cardiovascular system : Hypotension.
Central nervous system : Headache, disorientation, psychotic behaviour, including agitation, delusions and hallucinations may result from excess thiocyanate production.
Gastrointestinal system : Diarrhoea (usually from oral doses), osmotic disturbances. Nausea and vomiting may result from excess thiocyanate production.
Genitourinary system : Diuretic effects are possible.
Musculoskeletal system : Arthralgia, hyperreflexia and muscle cramps may result from excess thiocyanate production.
Ocular system : Blurred vision may result from excess thiocyanate production.
Ototoxicity : Tinnitus may occur from excess thiocyanate production.
OVERDOSAGE :
Overdose of sodium thiosulfate during treatment of cyanide poisoning results in thiocyanate toxicity. Symptoms of thiocyanate toxicity may be seen at serum thiocyanate concentrations above 10 mg/100 ml (1.72 mmol/l). Thiocyanate toxicity becomes life threatening at serum concentrations of 20 mg/100 ml (3.44 mmol/l).
The symptoms of thiocyanate toxicity include :
Arthralgias (pain in the joints), blurred vision, hyperreflexia, muscle cramps, nausea and vomiting, psychotic behaviour (agitation, delusions, hallucinations), tinnitus (ringing in the ears).
TREATMENT OF OVERDOSAGE :
Treatment of overdose is done by enhancing thiocyanate elimination using haemodialysis. Give supportive treatment as required.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the solution if translucent particles consistent with glass delamination are visible. Glass delamination can occur with high pH solutions when the surface glass from ampoule/vial separates into thin layers resulting in glass particles with a flaky appearance.
STORAGE :
Store below 25ºC, protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
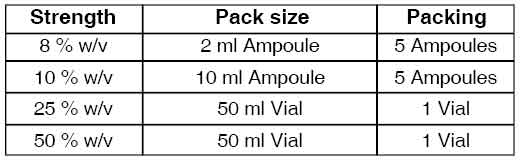
PRESENTATION :
SODIUM THIOSULFATE INJECTION is supplied in different strengths as following :
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular