300 mg/10 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
SODIUM NITRITE INJECTION USP (Sodium Nitrite) is an antidote for cyanide poisoning. It is generally used in conjunction with sodium thiosulfate, and often amyl nitrite, in the treatment of cyanide poisoning. Chemically, Sodium Nitrite is Nitrous acid, sodium salt. The molecular formula is NaNO2 and molecular weight is 69.00.
STRUCTURAL FORMULA :
Its structural formula is :
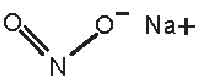
SODIUM NITRITE INJECTION USP is a sterile, clear, colourless solution filled in 10 ml amber vial.
COMPOSITION :
Each ml contains :
Sodium Nitrite USP 30 mg
Water for Injection USP q.s.
ACTIONS :
Sodium nitrite is an antidote for cyanide poisoning. Cyanide poisoning can be rapidly fatal. When hydrogen cyanide gas or large doses are taken, toxicity occurs within a few seconds, and death occurs within minutes. With smaller doses, toxicity occurs within minutes, and may include the following symptoms : constriction of the throat, nausea, vomiting, giddiness, headache, palpitations, hyperpnoea, then dyspnoea, bradycardia (which may be preceded by tachycardia), unconsciousness, violent convulsions followed by death. Sodium nitrite is generally used in conjunction with sodium thiosulfate, and often amyl nitrite, in the treatment of cyanide poisoning. Cyanide has a high affinity for ferric ions, and reacts readily with the ferric ion of mitochondrial cytochrome oxidase. Sodium nitrite reacts with haemoglobin to form methaemoglobin, and cyanide preferentially binds to methaemoglobin, restoring cytochrome oxidase activity. As cyanide dissociates from methaemoglobin, it is converted to the relatively non-toxic thiocyanate by the enzyme rhodanese. Sodium thiosulfate acts as a sulfur donor for rhodanese. Sodium nitrite also produces vasodilation by relaxing vascular smooth muscle.
PHARMACOKINETICS :
Sodium nitrite is rapidly absorbed following oral administration. After Intravenous administration the time to peak effect of sodium nitrite is 30 to 70 minutes. An Injection of 1 mg/kg sodium nitrite produces a peak methaemoglobin concentration of approximately 6 %.
INDICATIONS :
SODIUM NITRITE INJECTION USP in conjunction with sodium thiosulfate, is indicated for use as an antidote in the treatment of cyanide poisoning.
Administration :
SODIUM NITRITE INJECTION USP is administered by intravenous injection. Sodium thiosulfate should be administered immediately following the sodium nitrite dosage. Methaemoglobin concentration should be monitored and must not exceed 40 %.
Dosage :
Therapy should be instituted immediately based upon reasonable suspicion of cyanide toxicity. The characteristic smell of bitter almonds may not be obvious, and is not detectable by all individuals. Sodium nitrite should only be administered in several cases.
Adult dose :
The usual adult is 300 mg (10 ml of a 3 % solution administered intravenously at a rate of 75 to 150 mg/min (2.5 to 5 ml/min).
Paediatric dose :
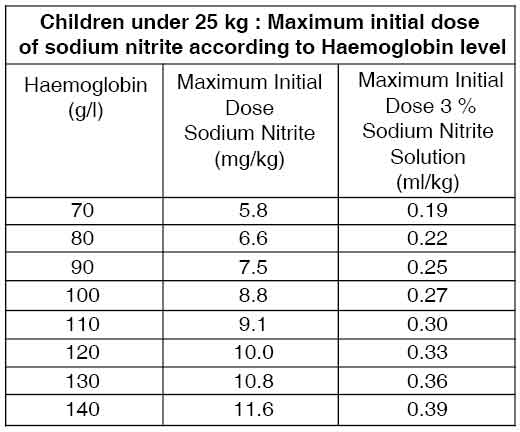
The usual paediatric dose is 4 mg/kg body weight (0.13 ml of a 3 % solution /kg body weight) [range 4.0 to 10 mg/kg body weight. 0.13 to 0.33 ml/kg body weight] or 180 to 240 mg/m2 ] administered at a rate of 75 to 150 mg/min [2.5 to 5 ml of a 3 % solution/min]. It is advisable to begin with doses at the lower end of the recommended range and increase to the desired effect. A maximum dose of 300 mg (10 ml of a 3 % solution) is recommended. For children under 25 kg, where anaemia is suspected, it is recommended that the dose of sodium nitrite be reduced relative to the haemoglobin measurement. The table below outlines a dosage regimen as a function of haemoglobin concentration.
In both adults and children, if symptoms of cyanide toxicity recur , treatment with half the original dose of sodium nitrite and sodium thiosulfate treatment may be repeated 30 minutes after the initial dose.
Use in Pregnancy :
Little is known about the effects of sodium nitrite on pregnancy and the foetus, however, problems in pregnancy have not been documented . Concerns about adverse effects on the foetus may have little relevance in the context of life threatening cyanide poisoning in the pregnant women. Animal experiments indicate that some sodium nitrite crosses the placenta and that foetal methaemoglobinaemia may be induced. The risk to the foetus from severe maternal cyanide poisoning should be evaluated against the risk of foetal methaemoglobinaemia.
Use in Lactation :
It is not known whether sodium nitrite is distributed into breast milk. Concerns about adverse effects on the breastfed infant may have little relevance in the context of life threatening cyanide poisoning in the mother. No animal studies have addressed the question of sodium nitrite excertion in breast milk or its possible effects on the nursing infant.
Patient Monitoring :
Methaemoglobin levels should be monitored during sodium nitrite treatment to avoid excessive methaemoglobin induction and should not exceed 40 %. Blood pressure should be monitored carefully during sodium nitrite administration, since hypotension may result if the rate of administration is too fast.
INTERACTIONS AND INCOMPATIBILITIES :
Sodium nitrite is reported to be incompatible with the following : acetanilide, antipyrine, caffeine citrate, chlorates, hypophosphites, iodides, mercury salts, morphine, oxidizing agents, permanganate, phenazone, sulfites, tannic acid, and vegetable astringent decoctions
infusions or tinctures.
SIDE EFFECTS :
SODIUM NITRITE INJECTION USP may cause nausea and vomiting , abdominal pain, dizziness, headache, flushing, cyanosis, tachypnoea, and dyspnoea, vasodilation resulting in syncope, hypotension, and tachycardia may occur. Overdosage may result in cardiovascular collapse, coma, convulsions, and death. Ionised nitrite readily oxidise haemoglobin to methaemoglobin, causing methaemoglobinaemia. SODIUM NITRITE INJECTION USP is a precursor for the formation of nitrosamines, many of which are carcinogenic in animals, but a relationship with human cancer has not been established.
OVERDOSAGE :
Clinical features :
Overdose of sodium nitrite results in methaemoglobinaemia. Symptoms of methaemoglobinaemia may be seen at blood methaemoglobin concentrations of 15 %, but symptoms do not usually appear until the blood methaemoglobin concentration reaches 30 to 40 %. The symptoms of methaemoglobinaemia include cyanosis, headache, unusual tiredness or weakness, tachycardia, shortness of breath, extreme dizziness or fainting and coma. Cardiovascular collapse, convulsions and death may occur after sodium nitrite overdose. The mean lethal oral dose of sodium nitrite in adults is approximately 1 gm if no treatment is received, although survival after this dose has been reported.
TREATMENT OF OVERDOSAGE :
Treatment of overdose involves the following measures :
- Supportive and symptomatic treatment.
- Intravenous administration of Methylene blue (1 to 2 mg/kg body weight) over 5 to 10 minutes. The dose may be repeated after 1 hour if necessary, but the total dose should not exceed 7 mg/kg. Extreme caution should be exercised when administering Methylene blue to patients likely to have substantial amounts of cyanide bound to methaemoglobin because Methylene blue will increase the cyanide release.
- Oxygen and exchange transfusion should be considered when methaemoglobinaemia is severe.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 25°C (77°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
SODIUM NITRITE INJECTION USP is supplied as 300 mg of Sodium Nitrite USP in 10 ml aqueous solution.
Such one vial of 10 ml is packed in a Box.

 Cardiovascular
Cardiovascular