500 mg/20 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
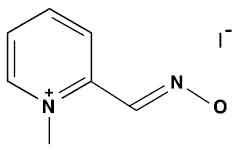
PRALIDOXIME IODIDE INJECTION is clear pale yellow solution filled in a vial of suitable size.
COMPOSITION :
Each ml Contains :
Pralidoxime Iodide 25 mg
Methylparaben USP 1.8 mg
Propylparaben USP 0.2 mg
(as preservatives)
Water for Injection USP q.s.
ACTIONS :
Pralidoxime Iodide is a cholinesterase reactivator. The principal action of pralidoxime is to reactivate cholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate pesticide or related compound. The destruction of accumulated acetylcholine can then proceed and neuromuscular junctions will again function normally. Pralidoxime also slows the process of “ageing” of phosphorylated cholinesterase to a non-reactivatable form and detoxifies certain organophosphates by direct chemical reaction. The drug has its most critical effect in relieving paralysis of the muscles of respiration. Because pralidoxime is less effective in relieving depression of the respiratory centre, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site. Pralidoxime relieves muscarinic signs and symptoms, salivation, bronchospasm etc. but this action is relatively unimportant since atropine is adequate for this purpose. It has been reported that the supplemental use of oxime cholinesterase reactivators (such as pralidoxime) reduces the incidence and severity of developmental defects in chick embryos exposed to such known teratogens as parathion, bidrin, carbachol and neostigmine. This protective effect of the oximes was shown to be dose related. PRALIDOXIME IODIDE INJECTION antagonizes the effects on the neuromuscular junction of the carbamate anticholinesterases, neostigmine, pyridostigmine and ambenonium, used in the treatment of myasthenia gravis. However, it is not nearly as effective as an antidote to these drugs as it is to the organophosphates.
PHARMACOKINETICS :
Pralidoxime is distributed throughout the extra cellular water; it is not bound to plasma protein. The drug is rapidly excreted in the urine partly unchanged, and partly as a metabolite produced by the liver. Consequently, pralidoxime is relatively short acting and repeated doses may be needed, especially where there is any evidence of continuing absorption of the poison. Pralidoxime Iodide is somewhat slowly absorbed from the gastrointestinal tract.
Distribution :
Distributed throughout the extra cellular water.
Protein binding :
Not bound to plasma proteins.
Biotransformation :
Metabolized largely by the liver.
Half-life :
Elimination half time is Approximately 1 to 2 hours.
Elimination :
Rapidly excreted in the urine, partly unchanged, and partly as a metabolite produced by the liver.
INDICATIONS :
PRALIDOXIME IODIDE INJECTION is indicated as an antidote :
- In the treatment of poisoning due to those pesticides and chemicals of the organophosphate class which have anticholinesterase activity and
- In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis.
- The principal indications for the use of pralidoxime are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness.
Administration :
For Intramuscular and Intravenous Infusion.
Dosage :
Pralidoxime Iodide Injection is extremely well tolerated in healthy volunteers. Single oral doses up to 7 g have produced minimal side effects. Intravenous doses are even better tolerated with doses as large as 40.5 g over a seven day period having no reported side effects.
Organophosphate Poisoning :
Pralidoxime is most effective if administered immediately after poisoning. Generally, little is accomplished if the drug is given more than 36 hours after termination of exposure. When the poison has been ingested, however, exposure may continue for some time due to slow absorption from the lower bowel, and fatal relapses have been reported after initial improvement. Continued administration for several days may be useful in such patients. Close supervision of the patient is indicated for at least 48 to 72 hours. If dermal exposure has occurred, clothing should be removed and the hair and skin washed thoroughly with sodium bicarbonate or alcohol as soon as possible. Diazepam may be given cautiously if convulsions are not controlled by atropine. Severe poisoning (coma, cyanosis, respiratory depression) requires intensive management. This includes the removal of secretions, airway management, the correction of acidosis and hypoxaemia. Atropine should be given as soon as possible after hypoxaemia is improved. Atropine should not be given in the presence of significant hypoxia due to the risk of atropine-induced ventricular fibrillation. In adults, atropine may be given intravenously in doses of 2 to 4 mg. This should be repeated at 5 to 10-minute intervals until full atropinization (secretions are inhibited) or signs of atropine toxicity appear (delirium, hyperthermia, muscle twitching). Some degree of atropinization should be maintained for at least 48 hours and until any depressed blood cholinesterase activity is reversed. Morphine, theophylline, aminophylline and succinylcholine are contraindicated. Tranquilizers of the reserpine or phenothiazine type are to be avoided. After the effects of atropine become apparent, PRALIDOXIME IODIDE INJECTION may be administered.
Adults.
Pralidoxime Iodide Injection is initially administered as slow intravenous dose of 1 to 2 gms. Signs of recovery (increasing consciousness, decreasing fasciculations and weakness) should occur rapidly. If the symptoms reappear, then an infusion of 2.5 % Pralidoxime Iodide is infused at a rate of 0.5 g/hour. A continuous infusion maintains adequate blood levels of pralidoxime better than intermittent injections. Intramuscular and oral administration is also effective, but intravenous is preferred because of rapidity of action. In severe cases, especially after ingestion of the poison, it may be desirable to monitor the effect of therapy electrocardiographically because of the possibility of heart block due to the anticholinesterase. Where the poison has been ingested, it is particularly important to take into account the likelihood of continuing absorption from the lower bowel since this constitutes new exposure. In such cases, additional doses of PRALIDOXIME IODIDE INJECTION may be needed every three to eight hours. In effect, the patient should be “titrated” with PRALIDOXIME IODIDE INJECTION as long as signs of poisoning recur. As in all cases of organophosphate poisoning, care should be taken to keep the patient under observation for at least 24 hours. If convulsions interfere with respiration, they may be controlled by the slow intravenous injection of diazepam, up to 20 mg in adults, or Sodium thiopentone (2 % solution) given intravenously.
In Children
The dose should be 20 to 50 mg per kg as intermittent doses required to alleviate symptoms. An infusion can be used after an initial dose has been given. One gram of PRALIDOXIME IODIDE INJECTION in 250 ml of 5 % dextrose and 0.2 % saline has been recommended. Treatment will be most effective if given within a few hours after poisoning has occurred. Usually, little will be accomplished if the drug is first administered more than 48 hours after exposure, but in severe poisoning, it is, nevertheless, indicated since occasionally patients have responded after such an interval. In the absence of severe gastrointestinal symptoms, resulting from the anticholinesterase intoxication, PRALIDOXIME IODIDE INJECTION may be administered orally in doses of 1 to 3 g every five hours.
CONTRAINDICATIONS :
The pralidoxime Iodide is contraindicated in patients who are hypersensitive to any component of the product.
WARNINGS :
Pralidoxime is not effective in the treatment of poisoning due to phosphorus, inorganic phosphates or organophosphates not having anticholinesterase activity.
PRECAUTIONS :
Pralidoxime has been very well tolerated in most cases, but it must be remembered that the desperate condition of the organophosphate-poisoned patient will generally mask such minor signs and symptoms as have been noted in normal subjects. Intravenous administration of PRALIDOXIME IODIDE INJECTION should be carried out slowly and, preferably, by infusion, since certain side effects such as tachycardia, laryngospasm and muscle rigidity have been attributed in a few cases to a too-rapid rate of injection.
PRALIDOXIME IODIDE INJECTION should be used with great caution in treating organophosphate overdosage in cases of myasthenia gravis since it may precipitate a myasthenic crisis. Because pralidoxime is excreted in the urine, a decrease in renal function will result in increased blood levels of the drug. Thus, the dosage of pralidoxime should be reduced in the presence of renal insufficiency. As the use of PRALIDOXIME IODIDE INJECTION for the treatment of poisoning of the carbamate class is relatively ineffective, and the fact that its use with carbamyl poisoning may lead to increased toxicity, PRALIDOXIME IODIDE INJECTION is not recommended for use in this class of poison.
Laboratory Tests :
Treatment of organophosphate poisoning should be instituted without waiting for the results of laboratory tests. Red blood cell, plasma cholinesterase and urinary paranitrophenol measurements (in the case of parathion exposure) may be helpful in confirming the diagnosis and following the course of the illness. A reduction in red blood cell cholinesterase concentration to below 50 % of normal has been seen only with organophosphate ester poisoning.
Pregnancy : Category C

 Cardiovascular
Cardiovascular