250 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
PENICILLAMINE CAPSULES USP (Penicillamine) acts as a chelating agent used in the treatment of Wilson’s disease. It is also used to reduce cystine excretion in cystinuria and to treat the patients with severe, active rheumatioid arthritis unresponsive to conventional
therapy.Chemically, penicillamine is D-Valine, 3-mercapto.The molecular formula is C5H11NO2S and molecular weight is 149.21.
STRUCTURAL FORMULA :
Its structural formula is :
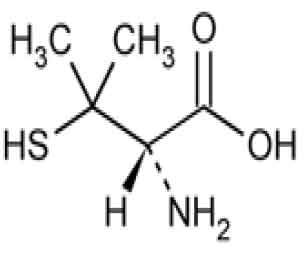
PENICILLAMINE CAPSULES USP are hard gelatin capsules containing off white powder.
COMPOSITION :
Each hard gelatin capsule contains :
Penicillamine USP 250 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Penicillamine is a chelating agent recommended for the removal of excess copper in patients with Wilson’s disease. From in vitro studies which indicate that one atom of copper combines with two molecules of penicillamine, it would appear that one gram of penicillamine should be followed by the excretion of about 200 milligrams of copper; however, the actual amount excreted is about one percent of this. Penicillamine also reduces excess cystine excretion in cystinuria. This is done, at least in part, by disulfide interchange between penicillamine and cystine, resulting in formation of penicillamine-cysteine disulfide, a substance that is much more soluble than cystine and is excreted readily. Penicillamine interferes with the formation of cross-links between tropocollagen molecules and cleaves them when newly formed.The mechanism of action of penicillamine in rheumatoid arthritis is unknown although it appears to suppress disease activity. Unlike cytotoxic immunosuppressants, penicillamine markedly lowers IgM rheumatoid factor but produces no significant depression in absolute levels of serum immunoglobulins. Also unlike cytotoxic immunosuppressants which act on both, penicillamine in vitro depresses T-cell activity but not B-cell activity.
In vitro, penicillamine dissociates macroglobulins (rheumatoid factor) although the relationship of the activity to its effect in rheumatoid arthritis is not known. In rheumatoid arthritis, the onset of therapeutic response to penicillamine may not be seen for two or three months.
In those patients who respond, however, the first evidence of suppression of symptoms such as pain, tenderness, and swelling is generally apparent within three months. The optimum duration of therapy has not been determined. If remissions occur, they may last from months to years, but usually require continued treatment. In all patients receiving penicillamine, it is important that penicillamine be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation by metal binding in the gastrointestinal tract.
PHARMACOKINETICS :
Penicillamine is absorbed rapidly but incompletely (40-70 %) from the gastrointestinal tract, with wide inter-individual variations. Food, antacids, and iron reduce absorption of the drug. The peak plasma concentration of penicillamine occurs 1-3 hours after ingestion. It is
approximately 1-2 mg/L after an oral dose of 250 mg. The drug appears in the plasma as free penicillamine, penicillamine disulfide, and cysteine-penicillamine disulfide. When prolonged treatment is stopped, there is a slow elimination phase lasting 4-6 days. More than 80 % of plasma penicillamine is bound to proteins. The drug also binds to erythrocytes and macrophages. A small fraction of the dose is metabolized in the liver to s-methyl-D-penicillamine. Drug excretion is primarily renal, mainly as disulfides.
INDICATIONS :
Rheumatoid arthritis juvenile rheumatoid arthritis. Symptomatic and asymptomatic Wilson’s disease. (hepatolenticular degeneration), (vital indication). Cystinuria (cystine stones), cystinoisis. Heavy metal poisoning (copper, gold, lead, mercury, cobalt, zinc) : In patients with lead poisoning penicillamine is prescribed to supplement or continue CaNa2EDTA treatment. In some instances penicillamine has successfully been used for arsenic poisoning in children. Primary billiary cirrhosis, chronic active hepatitis. Scleroderma, particularly if associated with interstitial pulmonary fibrosis. Benign hyperglobulinaemia purpura (Waldenstrom’s syndrome type I).
Administration :
In all patients receiving penicillamine, it is important that PENICILLAMINE CAPSULES USP be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food, or milk. Because penicillamine increases the requirement for pyridoxine, patients may require a daily supplement of pyridoxine.
Dosage :
Wilson’s Disease :
Optimal dosage can be determined by measurement of urinary copper excretion and the determination of free copper in the serum. The urine must be collected in copper-free glassware, and should be quantitatively analyzed for copper before and soon after initiation of therapy with PENICILLAMINE CAPSULES USP. Determination of 24-hour urinary copper excretion is of greatest value in the first week of therapy with penicillamine. In the absence of any drug reaction, a dose between 0.75 and 1.5 g that results in an initial 24-hour cupriuresis of over 2 mg should be continued for about three months, by which time the most reliable method of monitoring maintenance treatment is the determination of free copper in the serum. This equals the difference between quantitatively determined total copper and ceruloplasmin-copper. Adequately treated patients will usually have less than 10 mcg free copper/dL of serum. It is seldom necessary to exceed a dosage of 2 g/day. If the patient is intolerant to therapy with PENICILLAMINE CAPSULES USP, alternative treatment is trientine hydrochloride. In patients who cannot tolerate as much as 1 g/day initially, initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions.
Cystinuria :
It is recommended that PENICILLAMINE CAPSULES USP be used along with conventional therapy. By reducing urinary cystine, it decreases crystalluria and stone formation. In some instances, it has been reported to decrease the size of, and even to dissolve, stones already formed. The usual dosage of PENICILLAMINE CAPSULES USP in the treatment of cystinuria is 2 g/day for adults, with a range of 1 to 4 g/day. For paediatric patients, dosage can be based on 30 mg/kg/day. The total daily amount should be divided into four doses. If four equal doses are not feasible, give the larger portion at bedtime. If adverse reactions necessitate a reduction in dosage, it is important to retain the bedtime dose. Initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions. In addition to taking PENICILLAMINE CAPSULES USP, patients should drink copiously. It is especially important to drink about a pint of fluid at bedtime and another pint once
during the night when urine is more concentrated and more acid than during the day. The greater the fluid intake, the lower the required dosage of PENICILLAMINE CAPSULES USP.
Dosage must be individualized to an amount that limits cystine excretion to 100-200 mg/day in those with no history of stones, and below 100 mg/day in those who have had stone formation and/or pain. Thus, in determining dosage, the inherent tubular defect, the
patient’s size, age, and rate of growth, and his diet and water intake all must be taken into consideration. The standard nitroprusside cyanide test has been reported useful as a qualitative measure of the effective dose : Add 2 ml of freshly prepared 5 percent sodium cyanide to 5 ml of a 24-hour aliquot of protein-free urine and let stand ten minutes. Add 5 drops of freshly prepared 5 percent sodium nitroprusside and mix. Cystine will turn the mixture magenta. If the result is negative, it can be assumed that cystine excretion is less than 100 mg/g creatinine. Although penicillamine is rarely excreted unchanged, it also will turn the mixture magenta. If there is any question as to which substance is causing the reaction, a ferric chloride test can be done to eliminate doubt : Add 3 percent ferric chloride dropwise to the urine. Penicillamine will turn the urine an immediate and quickly fading blue. Cystine will not produce any change in appearance.
Rheumatoid Arthritis :
The principle rule of treatment with PENICILLAMINE CAPSULES USP in rheumatoid arthritis is patience. The onset of therapeutic response is typically delayed. Two or three months may be required before the first evidence of a clinical response is noted. When treatment with PENICILLAMINE CAPSULES USP has been interrupted because of adverse reactions or other reasons, the drug should be reintroduced cautiously by starting with a lower dosage and increasing slowly.
Initial Therapy :
The currently recommended dosage regimen in rheumatoid arthritis begins with a single daily dose of 125 mg or 250 mg which is thereafter increased at one to three month intervals, by 125 mg or 250 mg/day, as patient response and tolerance indicate. If a satisfactory remission of symptoms is achieved, the dose associated with the remission should be continued. If there is no improvement and there are no signs of potentially serious toxicity after two to three months of treatment with doses of 500-750 mg/day, increases of 250 mg/day at two to three month intervals may be continued until a satisfactory remission occurs or signs of toxicity develop. If there is no discernible improvement after three to four months of treatment with 1000 to 1500 mg of penicillamine/day, it may be assumed the patient will not
respond and PENICILLAMINE CAPSULES USP should be discontinued.
Maintenance Therapy :
The maintenance dosage of PENICILLAMINE CAPSULES USP must be individualized, and may require adjustment during the course of treatment. Many patients respond satisfactorily to a dosage within the 500-750 mg/day range. Some need less. Changes in
maintenance dosage levels may not be reflected clinically or in the erythrocyte sedimentation rate for two to three months after each dosage adjustment. Some patients will subsequently require an increase in the maintenance dosage to achieve maximal disease
suppression. In those patients who do respond, but who evidence incomplete suppression of their disease after the first six to nine months of treatment, the daily dosage of PENICILLAMINE CAPSULES USP may be increased by 125 mg or 250 mg/day at three-month intervals. It is unusual in current practice to employ a dosage in excess of 1 g/day, but up to 1.5 g/day has sometimes been required.
Management of Exacerbations :
During the course of treatment some patients may experience an exacerbation of disease activity following an initial good response. These may be self-limited and can subside within twelve weeks. They are usually controlled by the addition of non-steroidal anti-inflammatory drugs, and only if the patient has demonstrated a true “escape” phenomenon (as evidenced by failure of the flare to subside within this time period) should an increase in the maintenance dose ordinarily be considered. In the rheumatoid patient, migratory polyarthralgia due to penicillamine is extremely difficult to differentiate from an exacerbation of the rheumatoid arthritis. Discontinuance or a substantial reduction in dosage of PENICILLAMINE CAPSULES USP for up to several weeks will usually determine which of these processes is responsible for the arthralgia.
Duration of Therapy :
The optimum duration of therapy with PENICILLAMINE CAPSULES USP in rheumatoid arthritis has not been determined. If the patient has been in remission for six months or more, a gradual, stepwise dosage reduction in decrements of 125 mg or 250 mg/day at approximately three month intervals may be attempted.
Concomitant Drug Therapy :
PENICILLAMINE CAPSULES USP should not be used in patients who are receiving gold therapy, antimalarial or cytotoxic drugs, oxyphenbutazone, or phenylbutazone. Other measures, such as salicylates, other non-steroidal anti-inflammatory drugs, or systemic corticosteroids, may be continued when penicillamine is initiated. After improvement commences, analgesic and anti-inflammatory drugs may be slowly discontinued as symptoms permit. Steroid withdrawal must be done gradually, and many months of treatment with
PENICILLAMINE CAPSULES USP may be required before steroids can be completely eliminated. Dosage Frequency— Based on clinical experience dosages up to 500 mg/day can be given as a single daily dose. Dosages in excess of 500 mg/day should be administered in divided doses.
CONTRAINDICATIONS :
Except for the treatment of Wilson’s disease or certain patients with cystinuria, use of penicillamine during pregnancy is contraindicated. Although breast milk studies have not been reported in animals or humans, mothers on therapy with penicillamine should not nurse their infants. Patients with a history of penicillamine-related aplastic anaemia or agranulocytosis should not be restarted on penicillamine. Because of its potential for causing renal damage, penicillamine should not be administered to rheumatoid arthritis patients with a history or other evidence of renal insufficiency.
WARNINGS :
Physicians planning to use PENICILLAMINE CAPSULES USP should thoroughly familiarise themselves with potential toxicity and benefits. PENICILLAMINE CAPSULES USP should never be used casually. Each patient should remain constantly under the close
supervision of the physician. Patients should be warned to promptly report any symptoms suggesting toxicity. Patients should be instructed to report promptly fever, sore throat, chills, bruising or bleeding. PENICILLAMINE CAPSULES USP treatment should be
withdrawn if the total WBC falls below 3000 per cu. mm, neutrophils fall below 2000 or platelets below 120,000 or if a steady decline over three successive tests is observed, even though the counts remain within the normal range. A nephrotic syndrome may develop during therapy, and proteinuria may be a warning sign of its development. Close observation of patients is essential. In some cases proteinuria disappears with continued therapy; in other cases the medicine must be discontinued. When a patient develops proteinuria, the physician should ascertain that it is a symptom of the nephrotic syndrome and not transitory proteinuria unrelated to penicillamine. Withdraw treatment if albumin in the urine increases progressively to exceed 2 g per day. If successive tests demonstrate haematuria
discontinue PENICILLAMINE CAPSULES USP treatment immediately. Some patients may experience drug fever, a marked febrile response to penicillamine, usually in the second or third week following initiation of therapy. Drug fever may sometimes be accompanied by a macular cutaneous reaction.
If fever, or a reaction in the skin, blood or urine appears, PENICILLAMINE CAPSULES USP should be discontinued until the reaction subsides. After this, it should be reinstituted in patients with Wilson’s disease in a small dose that is gradually increased until full dosage is attained. Liver function tests are advisable every six months during the first year and a half of therapy. PENICILLAMINE CAPSULES USP increases the requirement of pyridoxine (Vitamin B6) and in rare cases causes central and peripheral nervous symptoms which could be due to pyridoxine deficiency. Some authorities recommend prophylactic administration of 25 mg pyridoxine daily. When PENICILLAMINE CAPSULES USP is used in cystinuria, an annual X-ray is advised. Cystine stones form rapidly, sometimes in six
months.
PRECAUTIONS :
Cross-allergy to penicillin and D–penicillamine may occur and penicillamine should be used with caution in the penicillin-hypersensitive patient. A neurological examination prior to therapy is advisable to distinguish pre-existing neurological disturbances from any which may arise during treatment. Should neurological abnormalities occur (excluding loss of taste) penicillamine should be withdrawn and other appropriate therapy initiated. Patients being treated with penicillamine should not be subjected to elective surgical procedures, especially vascular surgery, as penicillamine has the capacity to interfere with collagen cross links. Therapy with penicillamine should, if possible, be discontinued for at least six weeks prior to surgery. Some patients have experienced reversible optic neuritis, possibly related to pyridoxine deficiency, following the administration of a racemic mixture of penicillamine. However, symptoms of pyridoxine deficiency have not been reported in patients receiving penicillamine. Patients complaining of visual disturbances should undergo a full
ophthalmological examination. The frequency and severity of some of the adverse reactions are greatly reduced by gradual introduction of penicillamine.
Pregnancy : Category D
Controlled studies have not been done in pregnant women. However, birth defects have occurred in infants born to women who received penicillamine for rheumatoid arthritis or cystinuria during pregnancy. In rheumatoid arthritis : It is recommended that penicillamine not be used in pregnant women with rheumatoid arthritis. In cystinuria : It is recommended that penicillamine be avoided if possible in pregnant patients with cystinuria.In Wilson’s disease : Although birth defects have not been reported in infants of women receiving
penicillamine for Wilson’s disease, it is recommended that the daily dose be limited to 1 gram. Also, if cesarean section is planned, it is recommended that the daily dose be limited to 250 mg during the last 6 weeks of pregnancy and following surgery until wound healing is complete. Studies in animals have shown that penicillamine causes skeletal defects, cleft palates, and an increased number of resorptions when administered to rats in doses six times the maximum recommended human dose.
LACTATION :
It is not known whether penicillamine is distributed into breast milk. However, use is not recommended in the nursing mother.
INTERACTIONS AND INCOMPATIBILITIES :
Interactions :
Concomitant iron and penicillamine treatment : oral absorption of penicillamine may be reduced by concomitant administration of iron. Concomitant use of NSAIDs and other nephrotoxic drugs may increase the risk of renal damage. Concomitant gold and penicillamine treatment: concomitant use not recommended.
Incompatibilities :
Not applicable.
SIDE EFFECTS :
Signs of potential side effects, especially allergic reactions, fever (drug induced), pemphigus foliaceus or vulgaris, stomatitis, blood dyscrasias, glomerulopathy, obstructive bronchiolitis, exfoliative dermatitis, Goodpasture’s syndrome, jaundice, myasthenia gravis
syndrome, toxic epidermal necrosis, optic neuritis, pancreatitis, peptic ulcer reactivation, ringing or buzzing in ears, and SLE-like syndrome.
OVERDOSAGE :
No instances of adverse reactions to an overdose of penicillamine have been recorded and no specific measures are indicated.
TREATMENT OF OVERDOSAGE :
The treatment of D-penicillamine overdosage is nonspecific and essentially supportive. There is no known antidote.
STORAGE :
Store below 250C (770F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
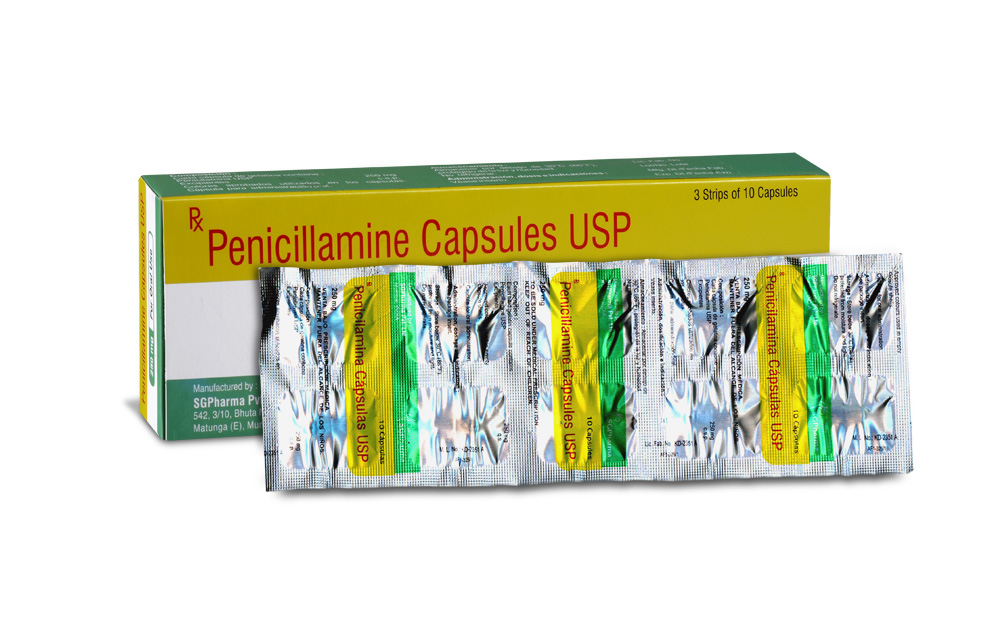
PENICILLAMINE CAPSULES USP contains Penicillamine USP 250 mg.
Box of 3 strips of 10 capsules.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular