50 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
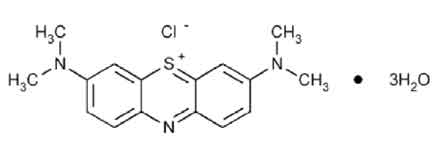
METHYLENE BLUE INJECTION USP (Methylene Blue) is used in treatment of methaemoglobinaemia. Chemically methylene blue is phenothiazin-5-ium,3,7-bis(dimethylamino)-,chloride, trihydrate. The molecular formula is C16H18CIN3S.3H2O and molecular weight is 373.90
STRUCTURAL FORMULA :
Its structural formula is :
METHYLENE BLUE INJECTION USP is clear, dark blue coloured solution filled in a amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Methylene Blue USP 10 mg
Water for Injection USP q.s.
Contains no preservatives.
ACTIONS :
In patients with methaemoglobinaemia, therapeutic doses of methylene blue can lower the levels of methaemoglobin in red blood cells. It activates a normally dormant reductase enzyme system that reduces the methylene blue to leucomethylene blue, which in turn is able to reduce methaemoglobin to haemoglobin. However, in large doses, methylene blue can itself produce methaemoglobinaemia and the methaemoglobin concentration should therefore be closely monitored during treatment. Methylene blue is not effective for the treatment of methaemoglobinaemia in patients with glucose-6-phosphate dehydrogenase deficiency as these patients have a diminished capacity to reduce methylene blue to leucomethylene blue. It is also potentially harmful as patients with glucose-6-phosphate dehydrogenase deficiency are particularly susceptible to the haemolytic anaemias induced by methylene blue. Methylene blue also possesses weak antiseptic and bacteriological staining properties and is reported to inhibit amine oxidase in tissues. The drug appears to bind irreversibly to viral nucleic acid and cause disruption of the virus molecule upon exposure to light. The use of methylene blue as a diagnostic aid is based on its ability to stain tissue. Any skin discolouration can be removed with hypochlorite solution.
PHARMACOKINETICS :
In tissues, methylene blue is rapidly reduced to leucomethylene blue, which is stabilised as an undetermined salt, complex, or combination form in the urine but not in the blood. About 75 % of an oral dose of methylene blue is excreted in the urine, primarily as leucomethylene blue, although a small proportion is excreted as the unchanged drug, while some is excreted via the bile.
INDICATIONS :
METHYLENE BLUE INJECTION USP is indicated in the treatment of acquired and idiopathic methaemoglobinaemia. METHYLENE BLUE INJECTION USP is used as a bacteriological stain, as a dye in diagnostic procedure, such as fistula detection and for the selective staining of certain body tissues during surgery. Methylene blue is also used intraamniotically to diagnose premature rupture of foetal membrane and to identify separate amniotic sacs in twin pregnancies.
Administration :
METHYLENE BLUE INJECTION USP administered by orally or by slow intravenous injection.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
METHYLENE BLUE INJECTION USP Usual adult and adolescent dose Methaemoglobinaemia Intravenous, 1 to 2 mg per kg of body weight or 25 to 50 mg per square meter of body surface area, administered over five minutes. The dose may be repeated after one hour if needed. Note : For treatment of Methaemoglobinaemia following overdose of agents in which there is prolonged or continuous methaemoglobin formation (e.g., dapsone), methylene blue may be administered by continuous intravenous infusion at a rate of 0.1 to 0.15 mg per kg of body weight per hour, following an initial dose of 1 to 2 mg per kg of body weight. Usual adult prescribing limits 7 mg per kg of body weight. A dose of 5 mg/kg diluted in 500 ml of saline (sodium chloride 0.9 %) or glucose 5 % infused over 1 hour has been used successfully to stain and identify the parathyroid glands. A suitable dilution for oral dosing would be 5 to 10 ml of the 1 % solution diluted to 100 to 200 ml with water for injection. The high volume is suggested to reduce the degree of gastrointestinal disturbances and dysuria. The dosage of methylene blue should be calculated on the basis of lean bodyweight. The use of methylene blue is not recommended in infants under 4 months of age. For continuous intravenous infusion, methylene blue should be admixed with a compatible solution, such as 0.9 % sodium chloride injection, to a final concentration of 0.05 %.
CONTRAINDICATIONS :
Methylene Blue Injection USP is contraindicated in the following circumstances :
1. Known hypersensitivity to the drug
2. Patients with severe renal impairment
3. Patients with glucose-6-phosphate dehydrogenase deficiency
4. Methaemoglobinaemia due to chlorate poisoning
5. Methaemoglobinaemia during treatment of cyanide poisoning.
Intrathecal and subcutaneous injection of methylene blue are also contraindicated, as they can result in neural damage (intrathecal administration) and necrotic abscess (subcutaneous administration). Intraspinal injection is contraindicated.
PRECAUTIONS :
Methaemoglobin concentration should be closely monitored during treatment as Methylene Blue can produce methaemoglobinaemia in large doses. Methylene Blue should be used with caution in the treatment of toxic methaemoglobinaemia; high doses can cause hemolytic anemias and patients with glucose-6-phosphate dehydrogenase (G6PD) deficiencies are particularly susceptible. A rapid disappearance of cyanosis in response to Methylene Blue would be expected within one hour but might not occur if the patient has erythrocyte G6PD or NADPH-diaphorase deficiency or if methaemoglobinaemia is due to the ingestion of compounds such as aniline or dapsone. A second dose has been recommended if cyanosis does not disappear within 1 hour of Methylene Blue administration but results of a study in animals and of a patient with aniline poisoning indicated that an increased dosage of Methylene Blue might be of no additional benefit and could be potentially dangerous in that it could enhance Heinz body formation. Methylene Blue should be used with caution in patients with glucose-6-phosphate dehydrogenase deficiency.
Use in Pregnancy : Category C
METHYLENE BLUE INJECTION USP should not be administered to pregnant women.
Nursing Mothers :
It is not known whether methylene blue is distributed into breast milk. However, problems in humans have not been documented.
Paediatric Use :
Extreme caution should be exercised when administering methylene blue to infants. During the first 4 months of life, infants have lower concentrations of the enzymes necessary for reducing methaemoglobin to haemoglobin, making these infants more susceptible to methaemoglobinaemia produced by high doses of methylene blue. Intraamniotic injection of methylene blue has resulted in haemolytic anaemia, hyperbilirubinaemia, methaemoglobinaemia, and deep blue staining of the newborn.
INTERACTIONS AND INCOMPATIBILITIES :
Incompatibilities :Methylene blue is reported to be incompatible with caustic alkalis, iodides and dichromates, and oxidising and reducing substances.
SIDE EFFECTS :
The following side effects have been reported after intravenous administration of methylene blue:
Gastrointestinal : nausea, vomiting, diarrhoea, abdominal pain, blue colour of faeces and saliva.
Haematologic : haemolysis (in glucose-6-phosphate dehydrogenase deficiency, or high doses), methaemoglobinaemia (after high doses).
Cardiovascular : hypertension, hypotension, arrhythmia, chest pain.
Body as a whole : profuse sweating.
Dermal : rash (blue macules, severe burning pain).
Nervous System : headache, dizziness, mental confusion.
Injection site : thrombophlebitis (resulting from high doses, if not adequately diluted - not more than 350 milligrams of methylene blue should be diluted in each 500 mL of infusion fluid), necrosis (if extravasation occurs).
Renal : blue color of urine. Oral administration may cause gastrointestinal disturbances and dysuria. Use of methylene blue for endoscopic tattoo has been associated with vascular necrosis, mucosal ulceration, mural necrosis, extramural fat necrosis and inflammatory changes in the colon. Injection of methylene blue into joint space has resulted in effusion in the treated joint.
OVERDOSAGE :
No specific information is available. However, large doses of methylene blue can produce methaemoglobinaemia. Side effects seen with high doses include chest pain, dyspnoea, restlessness, apprehension, tremors and a sense of oppression. Large doses are irritant to the urinary tract. In addition, it can produce a mild haemolysis with moderate hyperbilirubinaemia, reticulosis and slight anaemia. Rarely, however, severe haemolytic anaemia with Heinz body formation has resulted. Methylene blue in large doses could cause a blue discolouration of the skin after methaemoglobin levels have returned to normal.
TREATMENT OF OVERDOSAGE :
There is no specific antidote for methylene blue overdose. Treatment is symptomatic and supportive. In severe and refractory cases of methaemoglobinaemia, blood transfusions and even exchange transfusions and (possibly) hyperbaric oxygen may be the only alternative available.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 25°C (77°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
Methylene Blue Injection USP is supplied as 50 mg of Methylene blue USP in 5 ml aqueous solution.
Such 5 ampoules of 5 ml are packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular