10 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
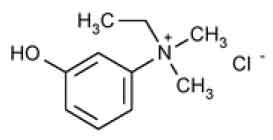
EDROPHONIUM CHLORIDE INJECTION USP (Edrophonium Chloride) is a short and rapid-acting cholinergic drug. Chemically, Edrophonium Chloride is ethyl(m-hydroxyphenyl) dimethylammonium chloride. The molecular formula is C10H16ClNO and molecular weight is 201.70.
STRUCTURAL FORMULA :
Its structural formula is :
EDROPHONIUM CHLORIDE INJECTION USP is a clear colourless sterile solution filled in ampoule of suitable size.
COMPOSITION :
Each ml contains :
Edrophonium Chloride USP 10 mg
Phenol USP 0.45 % w/v
(as preservative)
Water for Injection USP q.s.
ACTIONS :
Cholinergic (cholinesterase inhibitor) :
Inhibits destruction of acetylcholine by acetylcholinesterase, thereby facilitating transmission of impulses across the myoneural junction.
Diagnostic aid (myasthenia gravis) :
By prolonging the duration of action of acetylcholine at the motor end plate, edrophonium transiently increases muscle strength in patients with myasthenia gravis, whereas patients with other disorders develop either no increase in strength or even a slight weakness and possibly fasciculations.
Antidote (to nondepolarizing neuromuscular block) :
Since nondepolarizing neuromuscular blocking agents combine reversibly with the receptors, preventing access of acetylcholine, antagonism can be overcome by increasing the amount of agonist at the receptors; therefore, muscle paralysis induced by nondepolarizing neuromuscular blocking agents is reversed by edrophonium, which increases the concentration of acetylcholine at the receptors.
PHARMACOKINETICS :
Distribution : The volume of distribution (Vol D) of edrophonium is 1.1 to 0.2 L per kg.
Half-life : Distribution - 7 to 12 minutes.
Elimination - 33 to 110 minutes.
Onset of action : Intramuscular - 2 to 10 minutes.
Intravenous - Within 30 to 60 seconds. Onset of reversal of muscle relaxant-induced depression in twitch tension occurs within 3 minutes.
Time to peak effect : Following a 0.5 to 1 mg per kg of body weight (mg/kg) intravenous dose Within 1.2 minutes.
INDICATIONS :
EDROPHONIUM CHLORIDE INJECTION USP is recommended for the differential diagnosis of myasthenia gravis and as an adjunct in the evaluation of treatment requirements in this disease. It may also be used for evaluating emergency treatment in myasthenic crises. Because of its brief duration of action, it is not recommended for maintenance therapy in myasthenia gravis. EDROPHONIUM CHLORIDE INJECTION USP is also useful whenever a curare antagonist is needed to reverse the neuromuscular block produced by curare, tubocurarine, gallamine triethiodide or dimethyl-tubocurarine. It is not effective against decamethonium bromide and succinylcholine chloride. It may be used adjunctively in the treatment of respiratory depression caused by curare overdosage.
Administration :
FOR I.M. / I.V. USE ONLY.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
EDROPHONIUM CHLORIDE INJECTION USP Test in the Differential Diagnosis of Myasthenia Gravis.
Intravenous Dosage (Adults) :
A tuberculin syringe containing 1 ml (10 mg) of EDROPHONIUM CHLORIDE INJECTION USP is prepared with an intravenous needle, and 0.2 ml (2 mg) is injected intravenously within 15 to 30 seconds. The needle is left in situ. Only if no reaction occurs after 45 seconds is the remaining 0.8 ml (8 mg) injected. If a cholinergic reaction (muscarinic side effects, skeletal muscle fasciculations and increased muscle weakness) occurs after injection of 0.2 ml (2 mg), the test is discontinued and atropine sulfate, 0.4 mg to 0.5 mg, is administered intravenously. After one-half hour the test may be repeated.
Intramuscular Dosage (Adults) :
In adults with inaccessible veins, dosage for intramuscular injection is 1 ml (10 mg) of EDROPHONIUM CHLORIDE INJECTION USP. Subjects who demonstrate hyperreactivity to this injection (cholinergic reaction), should be retested after one-half hour with 0.2 ml (2 mg) of EDROPHONIUM CHLORIDE INJECTION USP intramuscularly to rule out false-negative reactions.
Dosage in paediatric Patients :
The intravenous testing dose of EDROPHONIUM CHLORIDE INJECTION USP in paediatric patients weighing up to 34.01 kg is 0.1 ml (1 mg); above this weight, the dose is 0.2 ml (2 mg). If there is no response after 45 seconds, it may be titrated up to 0.5 ml (5 mg) in paediatric patients under 34.01 kg, given in increments of 0.1 ml (1 mg) every 30 to 45 seconds and up to 1 ml (10 mg) in heavier patients. In infants, the recommended dose is 0.05 ml (0.5 mg). Because of technical difficulty with intravenous injection in paediatric patients, the intramuscular route may be used. In paediatric patients weighing up to 34.01 kg, 0.2 ml (2 mg) is injected intramuscularly. In paediatric patients weighing more than 34.01 kg, 0.5 ml (5 mg) is injected intramuscularly. All signs which would appear with the intravenous
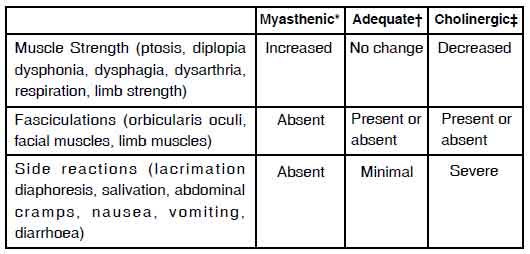
test appear with the intramuscular test except that there is a delay of 2 to 10 minutes before a reaction is noted. EDROPHONIUM CHLORIDE INJECTION USP Test for Evaluation of Treatment Requirements in Myasthenia Gravis The recommended dose is 0.1 ml to 0.2 ml (1 mg to 2 mg) of EDROPHONIUM CHLORIDE INJECTION USP, administered intravenously 1 hour after oral intake of the drug being used in treatment. Response will be myasthenic in the undertreated patient, adequate in the controlled patient, and cholinergic in the
overtreated patient. Responses to EDROPHONIUM CHLORIDE INJECTION USP in myasthenic and nonmyasthenic individuals are summarized in the following chart.
EDROPHONIUM CHLORIDE INJECTION USP Test in Crisis
The term crisis is applied to the myasthenic whenever severe respiratory distress with objective ventilatory inadequacy occurs and the response to medication is not predictable. This state may be secondary to a sudden increase in severity of myasthenia gravis
(myasthenic crisis), or to over treatment with anticholinesterase drugs (cholinergic crisis). When a patient is apneic, controlled ventilation must be secured immediately in order to avoid cardiac arrest and irreversible central nervous system damage. No attempt is made to test with EDROPHONIUM CHLORIDE INJECTION USP until respiratory exchange is adequate.
Dosage used at this time is most important
If the patient is cholinergic, EDROPHONIUM CHLORIDE INJECTION USP will cause increased oropharyngeal secretions and further weakness in the muscles of respiration. If the crisis is myasthenic, the test clearly improves respiration and the patient can be treated with longer-acting intravenous anticholinesterase medication. When the test is performed, there should not be more than 0.2 ml (2 mg) EDROPHONIUM CHLORIDE INJECTION USP in the syringe. An intravenous dose of 0.1 ml (1 mg) is given initially. The patient’s heart action is carefully observed. If, after an interval of 1 minute, this dose does not further impair the patient, the remaining 0.1 ml (1 mg) can be injected. If no clear improvement of respiration occurs after 0.2 ml (2 mg) dose, it is usually wisest to discontinue all
anticholinesterase drug therapy and secure controlled ventilation by tracheostomy with assisted respiration.
For Use as a Curare Antagonist
EDROPHONIUM CHLORIDE INJECTION USP should be administered by intravenous injection in 1 ml (10 mg) doses given slowly over a period of 30 to 45 seconds so that the onset of cholinergic reaction can be detected. This dosage may be repeated whenever necessary. The maximal dose for any one patient should be 4 ml (40 mg). Because of its brief effect, EDROPHONIUM CHLORIDE INJECTION USP should not be given prior to the administration of curare, tubocurarine, gallamine triethiodide or dimethyl-tubocurarine : it should be used at the time when its effect is needed. When given to counteract curare overdosage, the effect of each dose on the respiration should be carefully observed before it is repeated, and assisted ventilation should always be employed.
CONTRAINDICATIONS :
Known hypersensitivity to anticholinesterase agents; intestinal and urinary obstructions of mechanical type.
WARNINGS :
Whenever anticholinesterase drugs are used for testing, a syringe containing 1 mg of atropine sulfate should be immediately available to be given in aliquots intravenously to counteract severe cholinergic reactions which may occur in the hypersensitive individual, whether he is normal or myasthenic. EDROPHONIUM CHLORIDE INJECTION USP should be used with caution in patients with bronchial asthma or cardiac dysrhythmias. The transient bradycardia which sometimes occurs can be relieved by atropine sulfate. Isolated instances of cardiac and respiratory arrest following administration of EDROPHONIUM CHLORIDE INJECTION USP have been reported. It is postulated that these are vagotonic effects. EDROPHONIUM CHLORIDE INJECTION USP contains sodium sulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS :
Patients may develop “anticholinesterase insensitivity” for brief or prolonged periods. During these periods the patients should be carefully monitored and may need respiratory assistance. Dosages of anticholinesterase drugs should be reduced or withheld until patients again become sensitive to them.
Preganacy : Category C
The safety of EDROPHONIUM CHLORIDE INJECTION USP during pregnancy or lactation has not been established. Although the possible hazards to mother and child must be weighed against the potential benefits in every case, experience with EDROPHONIUM CHLORIDE INJECTION USP in pregnant patients with myasthenia gravis has revealed no untoward effect of the drug on the course of pregnancy.
Nursing mothers :
There is no information on the excretion of EDROPHONIUM CHLORIDE INJECTION USP into breast milk. Although only negligible amounts would be expected to be present, due regard should be paid to possible effects on the breast-feeding infant.
Paediatrics :
A study performed in 4 infants and 12 children did not show any paediatrics-specific problems that would limit the usefulness of edrophonium for neuromuscular blockade in children. Caution and careful monitoring are recommended.
INTERACTIONS AND INCOMPATIBILITIES :
The following drug interactions and/or related problems have been selected on the basis of their potential clinical significance (possible mechanism in parentheses where appropriate) not necessarily inclusive : Cholinesterase inhibitors, other, including antimyasthenics,
demecarium, echothiophate, and isoflurophate and possibly topical malathion in excessive quantities. (caution is recommended when administering edrophonium to patients with symptoms of myasthenic weakness who are also receiving these medications, since symptoms of cholinergic crisis [overdosage] may be similar to those occurring with myasthenic crisis [underdosage], and the patient’s condition may be worsened by use of edrophonium). Digitalis glycosides (when used concurrently with edrophonium, the additive vagomimetic effects may cause excessive bradycardia). Neuromuscular blocking agents (Phase I block of depolarizing neuromuscular blocking agents such as succinylcholine may be prolonged when these medications are used concurrently with edrophonium; however, if these blocking agents have been used over a prolonged period of time and the depolarization block has changed to a nondepolarization block, edrophonium may reverse the nondepolarization block) (Effects of many nondepolarizing neuromuscular blocking agents are antagonized by edrophonium, especially moderate degrees of residual neuromuscular blockade; profound levels of neuromuscular blockade may be better antagonized by other agents such as neostigmine)
SIDE EFFECTS :
Careful observation should be made for severe cholinergic reactions in the hyperreactive individual. The myasthenic patient in crisis who is being tested with edrophonium should be observed for bradycardia or cardiac standstill and cholinergic reactions if an overdose is given. The following reactions common to anticholinesterase agents may occur, although not all of these reactions have been reported with the administration of edrophonium, probably because of its short duration of action and limited indications :
Eye : Increased lacrimation, pupillary constriction, spasm of accommodation, diplopia, conjunctival hyperaemia.
CNS : Convulsions, dysarthria, dysphonia, dysphagia.
Respiratory : Increased tracheobronchial secretions, laryngospasm, bronchiolar constriction, paralysis of muscles of respiration, central respiratory paralysis.
Cardiac : Arrhythmias (especially bradycardia), fall in cardiac output leading to hypotension.
G.I. : Increased salivary, gastric and intestinal secretion, nausea, vomiting, increased peristalsis, diarrhoea, abdominal cramps.
Skeletal Muscle : Weakness, fasciculations.
Miscellaneous : Increased urinary frequency and incontinence, diaphoresis.
OVERDOSAGE :
With drugs of this type, muscarine-like symptoms (nausea, vomiting, diarrhoea, sweating, increased bronchial and salivary secretions and bradycardia) often appear with overdosage (cholinergic crisis). An important complication that can arise is obstruction of the airway by bronchial secretions. These may be managed with suction (especially if tracheostomy has been performed) and by the use of atropine. Many experts have advocated a wide range of dosages of atropine (for EDROPHONIUM CHLORIDE INJECTION USP, see atropine
dosage below),but if there are copious secretions, up to 1.2 mg intravenously may be given initially and repeated every 20 minutes until secretions are controlled. Signs of atropine overdosage such as dry mouth, flush and tachycardia should be avoided as tenacious secretions and bronchial plugs may form. A total dose of atropine of 5 to 10 mg or even more may be required.
TREATMENT OF OVERDOSAGE :
The following steps should be taken in the management of overdosage of EDROPHONIUM CHLORIDE INJECTION USP :
1. Adequate respiratory exchange should be maintained by assuring an open airway and the use of assisted respiration augmented by oxygen.
2. Cardiac function should be monitored until complete stabilization has been achieved.
3. Atropine sulfate in doses of 0.4 to 0.5 mg should be administered intravenously. This may be repeated every 3 to 10 minutes. Because of the short duration of action of EDROPHONIUM CHLORIDE INJECTION USP the total dose required will seldom exceed 2 mg.
4. If convulsions occur or shock is present, appropriate measures should be instituted.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from date of manufacture.
PRESENTATION :
EDROPHONIUM CHLORIDE INJECTION USP is supplied as 10 mg Edrophonium Chloride USP in 1 ml Ampoule.
Single ampoule per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular