100 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
DIMERCAPROL INJECTION (Dimercaprol) is an antidote in heavy metal poisoning; metal complexing agent. Chemically, dimercaprol is (RS)-2, 3-dimercaptopropanol. The molecular formula is C3H8OS2 and molecular weight is 124.22.
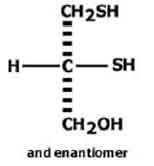
STRUCTURAL FORMULA :
Its structural formula is :
DIMERCAPROL INJECTION is a clear, yellow, viscous solution, having a pungent, disagreeable odour filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Dimercaprol I.P. 50 mg
Arachis Oil I.P. q.s.
ACTIONS :
Dimercaprol is a chelating agent used in the treatment of acute poisoning by heavy metals. The sulphydryl groups of dimercaprol compete with endogenous sulphydryl groups on proteins such as enzymes to combine with these metals; chelation by dimercaprol therefore prevents or reverses any inhibition of the sulphydryl enzymes by the metal and the dimercaprol-metal complex formed is readily excreted by the kidney. In addition, in lead toxicity, dimercaprol causes fast but short-lived reduction in lead concentration in red blood cells and CNS and effects a greater total lead excretion (urinary and faecal) than edetate calcium disodium because of its high faecal lead output. The addition of equimolar amounts of dimercaprol to edetate calcium disodium doubles the ratio of chelants to lead, thus providing the molar excess of chelating agent that is necessary for significant heavy metal excretion.
PHARMACOKINETICS :
Distribution :
All tissues, including the brain, but mainly in the intracellular space. The highest concentrations are in the liver and kidneys.
Biotransformation :
About 50 % rapidly metabolized to inactive metabolites.
Onset of action :
30 minutes.
Time to peak concentration :
30 to 60 minutes after intramuscular administration.
Duration of action :
About 4 hours. Frequent doses at 3- to 4- hour intervals over prolonged periods are necessary to maintain therapeutic effect.
Elimination :
50 % as the dimercaprol-metal complex, via the renal and biliary tracts; as metabolites, in the urine; metabolism and excretion are usually complete within 6 to 24 hours.
INDICATIONS :
DIMERCAPROL INJECTION is indicated in the treatment of arsenic, gold and mercury poisoning. It is indicated in acute lead poisoning when used concomitantly with Edetate Calcium Disodium Injection USP.
DIMERCAPROL INJECTION is effective for use in acute poisoning by mercury salts if therapy is begun within one or two hours following ingestion. It is not very effective for chronic mercury poisoning.
DIMERCAPROL INJECTION is of questionable value in poisoning caused by other heavy metals such as antimony and bismuth. It should not be used in iron, cadmium or selenium poisoning because the resulting dimercaprol-metal complexes are more toxic than the metal alone, especially to the kidneys. Also useful in hepatolenticular degeneration (Wilson’s disease).
Administration :
By deep intramuscular injection only.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
For mild arsenic or gold poisoning, 2.5 mg/kg of body weight four times daily for two days, two times on the third day and once daily thereafter for ten days; for severe arsenic or gold poisoning, 3 mg/kg every four hours for two-days, four times on the third day then twice daily thereafter for ten days. For mercury poisoning, 5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days. For acute lead encephalopathy, 4 mg/kg body weight is given alone in the first dose and thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site. For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose. Treatment is maintained for two to seven days depending on clinical response. Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals. Other supportive measures should always be used in conjunction with DIMERCAPROL INJECTION therapy. Wilson’s Disease : 300 mg daily for 10 days every 2nd month for long period.
CONTRAINDICATIONS :
DIMERCAPROL INJECTION is contra-indicated in poisoning by iron, cadmium or selenium, in the presence of impaired hepatic function unless due to arsenic poisoning and in patients hypersensitive to dimercaprol.
WARNINGS :
The drug should be discontinued or used only with extreme caution if acute renal insufficiency develops during therapy. There may be local pain at the site of the injection. A reaction apparently peculiar to children is fever which may persist during therapy. It occurs in approximately 30 % of children. A transient reduction of the percentage of polymorphonuclear leukocytes may also be observed.
PRECAUTIONS :
Dimercaprol should be used with care in patients with hypertension or renal impairment. It should be discontinued or continued with extreme caution, if acute renal insufficiency develops during therapy. Alkalinisation of the urine may protect the kidney during therapy by stabilising the dimercaprol-metal complex. Dimercaprol should not be used in patients with hepatic impairment unless due to arsenic poisoning. It should not be used in the treatment of poisoning due to cadmium, iron or selenium as the dimercaprol-metal complexes formed are more toxic than the metals themselves. Because the dimercaprol-metal complex breaks down easily in an acid medium, production of an alkaline urine affords protection to the kidney during therapy. Medicinal iron should not be administered to patients under therapy with DIMERCAPROL INJECTION. DIMERCAPROL INJECTION is formulated with arachis oil. Arachis oil may cause allergic reactions in some individuals. Physicians should use caution in prescribing DIMERCAPROL INJECTION for arachis-sensitive patients. Medication and equipment necessary to treat allergic reactions should be available if the product is administered to arachis-allergic patients.
Pregnancy : Category C
Animal reproduction studies have not been conducted with DIMERCAPROL INJECTION. It is also not known whether DIMERCAPROL INJECTION can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. DIMERCAPROL INJECTION should be given to a pregnant woman only if clearly needed.
Lactation :
It is not known whether this drug is excreted in human milk. However, because many drugs are excreted in human milk, caution should be exercised when DIMERCAPROL INJECTION is administered to a nursing woman.
Paediatric Use :
Fever, which appears after the second or third dose of dimercaprol, persists throughout therapy and disappears upon withdrawal of therapy, is more likely to occur in children than in adults. A transient reduction of polymorphonuclear leukocytes may also be seen.
DRUG INTERACTIONS :
Iron supplements should not be given during dimercaprol therapy as toxic dimercaprol-metal complexes are formed.
SIDE EFFECTS :
Side effects are relatively frequent, but at the therapeutic dosage employed, are seldom severe enough to warrant cessation of treatment and are almost invariably reversible. There is some evidence to indicate that 30 - 60 mg of ephedrine sulphate by mouth, given half an hour before each injection of dimercaprol, will reduce these reactions. Also, a minimum interval of four hours between doses appears to reduce side effects. Dimercaprol may cause the following side effects, particularly at the higher dosage levels : elevation of blood pressure accompanied by tachycardia, nausea and possibly vomiting, burning sensation of the lips, mouth, throat and eyes, salivation and lacrimation, conjunctivitis, rhinorrhoea, muscle pain and spasm, abdominal pain, headache, tingling of the hands and other extremities, a feeling of constriction in the chest and throat, sweating of the forehead and hands. Local pain may occur at the site of injection and gluteal abscess has occasionally been encountered. A side effect apparently peculiar to children is a fever which develops after the second or third injection and persists until treatment with dimercaprol is terminated.
OVERDOSAGE :
Symptoms of overdosage include malaise, nausea, vomiting, lacrimation and salivation, burning sensation of lips, mouth, throat and eyes with headache. A sense of constriction of the throat and chest. Increased blood pressure maximal after 15 - 20 minutes. Transient effects lasting about four hours.
TREATMENT OF OVERDOSAGE :
Treatment consists of the subcutaneous administration of diphenhydramine 50 mg or ephedrine 30 mg or ephedrine in a dosage of 30 - 60 mg orally if time permits.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C, protected from light.
Do not refrigerate.
Store in light resistant containers.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DIMERCAPROL INJECTION is supplied as 100 mg dimercaprol in 2 ml oily solution.
Such 2 ampoules of 2 ml are packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular