98 % v/v /10 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
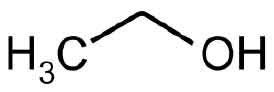
DEHYDRATED ALCOHOL INJECTION USP contains Dehydrated alcohol. Dehydrated alcohol is hypobaric in relation to the cerebrospinal fluid. It is injected proximate to nerve tissues and into spinal subarachnoid spaces to produce degeneration of nerve function (neurolysis) for control of chronic pain.Chemically Dehydrated alcohol is Ethanol. The molecular formula is C2H6O and molecular weight is 46.07
STRUCTURAL FORMULA :
Its structural formula is :
DEHYDRATED ALCOHOL INJECTION USP is a clear, colourless, sterile solution.
COMPOSITION :
Each ml contains :
Dehydrated Alcohol USP 98 % v/v
Water for Injection USP q.s.
Contains no preservatives.
ACTIONS :
Alcohol produces injury to tissue cells by dehydration and precipitation of protoplasm. When alcohol is injected in close proximity to nerve tissues, it produces neuritis and nerve degeneration (neurolysis). Deliberate injury to selected spinal nerves, peripheral nerves, or ganglia by injection of alcohol results in more or less enduring block of sensory, motor and autonomic function. The injection of alcohol used for therapeutic neurolysis involves amounts too small to produce significant systemic effects of ethanol. 90 to 98 % of ethyl alcohol that enters the body is completely oxidized.
PHARMACOKINETICS :
Ethanol is rapidly distributed throughout body fluids, with a volume of distribution of approximately 0.6 l/kg. It readily crosses the placenta. 90 to 98 % of ethanol is metabolised in the liver. The majority of this is metabolised via alcohol dehydrogenase to acetaldehyde, which is further metabolised by aldehyde dehydrogenase to acetic acid. A second pathway, which operates optimally at higher ethanol concentrations, oxidises ethanol via a microsomal oxidising system, which is induced by repeated exposure to ethanol. The remaining 2 % to 10 % of ethanol is excreted directly via the kidneys, lungs, sweat or other bodily secretions. Ethanol is also excreted in breast milk.
INDICATIONS :
DEHYDRATED ALCOHOL INJECTION USP is indicated for therapeutic neurolysis of nerves or ganglia for the relief of intractable chronic pain in such conditions as inoperable cancer and trigeminal neuralgia (ticdouloureux), in patients for whom neurosurgical procedures are contraindicated. Relief of trigeminal neuralgia usually is only temporary. Other conditions for which injections of alcohol has been reported include glossopharyngeal neuralgia, angina pectoris and severe claudication due to peripheral vascular insufficiency. DEHYDRATED ALCOHOL INJECTION USP concentrations of 40 to 50 % (prepared by appropriate dilution of dehydrated alcohol) have been used for epidural or individual motor nerve injections to control certain manifestations of cerebral palsy and spastic paraplegia. Similar concentrations also have been injected for celiac plexus block to relieve pain of inoperable upper abdominal cancer and have been injected intra-and subcutaneously for relief of intractable pruritus.
Administration : For Subarachnoid Injections
Dosage :
The dosage of DEHYDRATED ALCOHOL INJECTION USP for therapeutic nerve or ganglion block varies from as little as 0.05 to 0.5 ml in trigeminal neuralgia to 0.5 to 1.0 ml per interspace for subarachnoid injections. Doses larger than 1.5 ml are seldom required. All injections should be made slowly and only after all steps have been taken to insure precise placement of the alcohol. A 1.0 ml tuberculin syringe is desirable to facilitate accurate measurement of the dose. Separate needles should be used for injection of successive interspaces or other sites. Since DEHYDRATED ALCOHOL INJECTION USP is hypobaric as compared to spinal fluid, proper poisoning of the patient is essential to control localization of injections into the subarachnoid space. When lesser concentration of alcohol are used, larger volumes are usually injected. A dose of 2 ml of 45 % alcohol has been used for injecting individual motor nerves, or more 1.5 to 4.0 ml for epidural injection in children with spastic cerebral palsy; 50 ml of 50 % alcohol has been used for celiac plexus blockade
CONTRAINDICATIONS :
Subarachnoid injection of dehydrated alcohol is contraindicated in patients receiving anticoagulants because of the danger of bleeding.
WARNINGS :
DEHYDRATED ALCOHOL INJECTION USP is flammable liquid and should be kept cool and away from any heat source. Alcohol injections should be made with care to avoid unwanted tissue necrosis. Proper positioning of the patient is essential to control localization of injections of dehydrated alcohol into the subarachnoid spaces.
PRECAUTIONS :
It is sometimes advisable to make a trial injection of procaine or other local anesthetic prior to alcohol injection as a means of confirming accurate placement of the needle, and to decrease pain experienced during the procedure. X-ray visualization for precise placement also may be advisable. When used for selective sensory block within the subarachnoid space, it is essential to avoid contact of the alcohol with the anterior (motor) roots of the spinal nerve to be treated if motor paralysis is not desired. When peripheral nerves are injected, care should be taken that residual alcohol is not deposited along the needle track or in any other locations where tissue destruction is not wanted. Instances have been reported in which the pain resulting from post-injection neuritis was more severe than that existing before the injection.
Pregnancy : Category C.
Animal reproduction studies have not been conducted with dehydrated alcohol. It is also not known whether dehydrated alcohol can cause fetal harm when given to a pregnant woman or can affect reproduction capacity. Dehydrated alcohol should be given to a pregnant woman only if clearly needed.
Nursing mothers :
Breast feeding should be temporarily suspended during treatment with DEHYDRATED ALCOHOL INJECTION USP. Dehydrated alcohol is excreted in breast milk, and its safety in neonates has not been established.
INTERACTIONS AND INCOMPATIBILITIES :
The following drugs may interact pharmacokinetically with DEHYDRATED ALCOHOL INJECTION USP :
Drugs metabolised by the cytochrome P450 system :
DEHYDRATED ALCOHOL INJECTION USP may compete with other drugs metabolised by this enzyme system, potentially decreasing the clearance of both drugs. Chronic administration of DEHYDRATED ALCOHOL INJECTION USP may induce the cytochrome P450 enzyme system, potentially increasing the clearance of drugs metabolised by this system when alcohol is not present.
Disulfuram :
Disulfuram interferes with DEHYDRATED ALCOHOL INJECTION USP metabolism at the aldehyde stage, leading to unpleasant or, at larger concentrations, dangerous increase in aldehyde concentrations. Similar interactions may occur with cephalosporins, chlorpropamide, metronidazole, and tolbutamide.
Oral contraceptives :
Women taking oral contraceptives may have decreased elimination of DEHYDRATED ALCOHOL INJECTION USP.
The following drugs may interact pharmacodynamically with DEHYDRATED ALCOHOL INJECTION USP :
Central Nervous System depressants :
(such as hypnotics, muscle relaxants, opioid analgesics, antiepileptics, antidepressants and tranquilisers) : DEHYDRATED ALCOHOL INJECTION USP may enhance the depressant effects of these drugs.
Insulin :
DEHYDRATED ALCOHOL INJECTION USP may cause hypoglycaemic reactions in patients receiving insulin.
Oral anticoagulants :
DEHYDRATED ALCOHOL INJECTION USP may have variable effects on bleeding time in patients taking oral anticoagulants.
Sulphonylurea antidiabetic agents :
DEHYDRATED ALCOHOL INJECTION USP may cause hypoglycaemic reactions in patients receiving these agents.
Vasodilators :
DEHYDRATED ALCOHOL INJECTION USP may cause orthostatic hypotension in patients taking vasodilator agents or drugs with vasodilator action.
Vasopressin :
DEHYDRATED ALCOHOL INJECTION USP may decrease the antidiuretic effect of vasopressin.
SIDE EFFECTS :
The most commonly encountered side effects are post-injection neuritis with persistent pain, hyperesthesia and paresthesia. Subarachnoid neurolysis and lumbar sympathetic block may be followed by motor paralysis, bladder or rectal incontinence and impotence. Severe hypotension may follow celiac ganglion injection. Corneal anesthesia, meningitis or cranial nerve palsy may follow injection of the gasserian ganglion.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Excessive or faulty localization of injections may result in unwanted post-injection neuritis and/or tissue necrosis. In such cases, efforts should be directed toward dilution of deposited alcohol when feasible, relief of pain with analgesics and surgical intervention if indicated. Hypotension following celiac ganglion injection may be controlled with appropriate vasopressor agents. See PRECAUTIONS and SIDE EFFECTS. The LD50 oral dose in rats is 13.7 g/kg.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DEHYDRATED ALCOHOL INJECTION USP is supplied as 98 % v/v Dehydrated Alcohol USP in 10 ml solution.
Single vial pack.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular