300 mg, 200 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
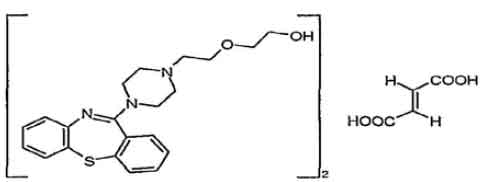
QUETIAPINE FUMARATE TABLETS I.P. (Quetiapine Fumarate) is antipsychotic. Chemically, Quetiapine Fumarate is 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol Fumarate. The molecular formula is (C21H25N3O2S)2,C4H4O4 and molecular weight is 883.10.
STRUCTURAL FORMULA :
Its structural formula is :
QUETIAPINE FUMARATE TABLETS I.P. 200 mg is white coloured, elongated, biconvex, film coated tablet, breakline on one side and plain on other side.
QUETIAPINE FUMARATE TABLETS I.P. 300 mg is white coloured, elongated, biconvex, film coated tablet, breakline on one side and plain on other side.
COMPOSITION :
Each film coated tablet contains :
Quetiapine Fumarate I.P.
equivalent to Quetiapine 200 mg
Excipients q.s.
Colour : Titanium Dioxide I.P.
Each film coated tablet contains :
Quetiapine Fumarate I.P.
equivalent to Quetiapine 300 mg
Excipients q.s.
Colour : Titanium Dioxide I.P.
ACTIONS :
Quetiapine Fumarate is an antagonist at multiple neurotransmitter receptors in the brain : serotonin 5HT1A and 5HT2 (IC50s =717 & 148nM respectively), dopamine D1 and D2 (IC50s =1268 & 329nM respectively), histamine H1 (IC50 =30nM), and adrenergic (alpha)1 and (alpha)2 receptors (IC50s = 94 & 271nM, respectively). Quetiapine has no appreciable affinity at cholinergic muscarinic and benzodiazepine receptors (IC50s > 5000 nM). The mechanism of action of Quetiapine, as with other drugs having efficacy in the treatment of schizophrenia and acute manic episodes associated with bipolar disorder, is unknown. However, it has been proposed that this drug’s efficacy in schizophrenia is mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism. Antagonism at receptors other than dopamine and 5HT2 with similar receptor affinities may explain some of the other effects of Quetiapine. Quetiapine Fumarate antagonism of histamine H1 receptors may explain the somnolence observed with this drug. Quetiapine Fumarate antagonism of adrenergic (alpha)1 receptors may explain the orthostatic hypotension observed with this drug.
PHARMACOKINETICS :
Quetiapine Fumarate activity is primarily due to the parent drug. The multiple-dose pharmacokinetics of quetiapine are dose-proportional within the proposed clinical dose range, and quetiapine accumulation is predictable upon multiple dosing. Elimination of quetiapine is mainly via hepatic metabolism with a mean terminal half-life of about 6 hours within the proposed clinical dose range. Steady-state concentrations are expected to be achieved within two days of dosing. Quetiapine is unlikely to interfere with the metabolism of drugs metabolized by cytochrome P450 enzymes.
Absorption :
Quetiapine Fumarate is rapidly absorbed after oral administration, reaching peak plasma concentrations in 1.5 hours. The tablet formulation is 100 % bioavailable relative to solution. The bioavailability of quetiapine is marginally affected by administration with food, with Cmax and AUC values increased by 25 % and 15 %, respectively.
Distribution :
Quetiapine Fumarate is widely distributed throughout the body with an apparent volume of distribution of 10 ± 4 L/kg. It is 83 % bound to plasma proteins at therapeutic concentrations. In vitro, quetiapine did not affect the binding of warfarin or diazepam to human serum albumin. In turn, neither warfarin nor diazepam altered the binding of quetiapine.
Metabolism and Elimination :
Following a single oral dose of 14C-quetiapine, less than 1 % of the administered dose was excreted as unchanged drug, indicating that quetiapine is highly metabolized. Approximately 73 % and 20 % of the dose was recovered in the urine and faeces, respectively.
Quetiapine is extensively metabolized by the liver. The major metabolic pathways are sulfoxidation to the sulfoxide metabolite and oxidation to the parent acid metabolite; both metabolites are pharmacologically inactive. In vitro studies using human liver microsomes revealed that the cytochrome P450 3A4 isoenzyme is involved in the metabolism of quetiapine to its major, but inactive, sulfoxide metabolite.
INDICATIONS :
QUETIAPINE FUMARATE TABLETS I.P. is indicated for the treatment of schizophrenia.
QUETIAPINE FUMARATE TABLETS I.P. is indicated for treatment of bipolar disorder :
• for the treatment of moderate to severe manic episodes in bipolar disorder.
• for the treatment of major depressive episodes in bipolar disorder.
• for the prevention of recurrence in patients with bipolar disorder, in patients whose manic or depressive episode has responded to quetiapine treatment.
Administration :
QUETIAPINE FUMARATE TABLETS I.P. is administered orally.
QUETIAPINE FUMARATE TABLETS I.P. may be administered with or without food.
Dosage :
Adults
For the treatment of schizophrenia :
QUETIAPINE FUMARATE TABLETS I.P. should be administered twice a day. The total daily dose for the first four days of therapy is 50 mg (Day 1), 100 mg (Day 2), 200 mg (Day 3) and 300 mg (Day 4). From Day 4 onwards, the dose should be titrated to the usual effective dose range of 300 to 450 mg/day. Depending on the clinical response and tolerability of the individual patient, the dose may be adjusted within the range 150 to 750 mg/day.
For the treatment of moderate to severe manic episodes associated with bipolar disorder :
QUETIAPINE FUMARATE TABLETS I.P. should be administered twice a day. The total daily dose for the first four days of therapy is 100 mg (Day 1), 200 mg (Day 2), 300 mg (Day 3) and 400 mg (Day 4). Further dosage adjustments up to 800 mg/day by Day 6 should be in increments of no greater than 200 mg per day. The dose may be adjusted depending on clinical response and tolerability of the individual patient, within the range of 200 to 800 mg per day. The usual effective dose is in the range of 400 to 800 mg per day.
For the treatment of depressive episodes in bipolar disorder :
QUETIAPINE FUMARATE TABLETS I.P. should be administered once daily at bedtime. The total daily dose for the first four days of therapy is 50 mg (Day 1), 100 mg (Day 2), 200 mg (Day 3) and 300 mg (Day 4). The recommended daily dose is 300 mg. In clinical trials, no additional benefit was seen in the 600 mg group compared to the 300 mg group. Individual patients may benefit from a 600 mg dose. Doses greater than 300 mg should be initiated by physicians experienced in treating bipolar disorder. In individual patients, in the event of tolerance concerns, clinical trials have indicated that dose reduction to a minimum of 200 mg could be considered.
For preventing recurrence in bipolar disorder :
For prevention of recurrence of manic, depressive and mixed episodes in bipolar disorder, patients who have responded to QUETIAPINE FUMARATE TABLETS I.P. for acute treatment of bipolar disorder should continue therapy at the same dose. The dose may then be adjusted depending on clinical response and tolerability of the individual patient, within the range of 300 to 800 mg/day administered twice daily. It is important that the lowest effective dose is used for maintenance therapy.
Elderly :
As with other antipsychotics and antidepressants, QUETIAPINE FUMARATE TABLETS I.P. should be used with caution in the elderly, especially during the initial dosing period. The rate of dose titration of quetiapine may need to be slower, and the daily therapeutic dose lower, than that used in younger patients, depending on the clinical response and tolerability of the individual patient. The mean plasma clearance of quetiapine was reduced by 30 % to 50 % in elderly patients when compared to younger patients. Efficacy and safety has not been evaluated in patients over 65 years with depressive episodes in the framework of bipolar disorder.
Children and adolescents :
QUETIAPINE FUMARATE TABLETS I.P. is not recommended for use in children and adolescents below 18 years of age, due to a lack of data to support use in this age group.
Renal impairment :
Dosage adjustment is not necessary in patients with renal impairment.
Hepatic impairment :
Quetiapine is extensively metabolised by the liver. Therefore, QUETIAPINE FUMARATE TABLETS I.P. should be used with caution in patients with known hepatic impairment, especially during the initial dosing period. Patients with hepatic impairment should be started with 25 mg/day. The dose can be increased in increments of 25 – 50 mg/day to an effective dose, depending on the clinical response and tolerability of the individual patient.
CONTRAINDICATIONS :
QUETIAPINE FUMARATE TABLETS I.P. are contraindicated in patients with known hypersensitivity to quetiapine or to any of the other ingredients in QUETIAPINE FUMARATE TABLETS I.P. Concomitant administration of cytochrome P450 3A4 inhibitors, such as HIV-protease inhibitors, azole-antifungal agents, erythromycin, clarithromycin and nefazodone, is contraindicated. QUETIAPINE FUMARATE TABLETS I.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome or lactase deficiency.
WARNINGS AND PRECAUTIONS :
As QUETIAPINE FUMARATE TABLETS I.P. has several indications, the safety profile should be considered with respect to the individual patient’s diagnosis and the dose being administered.
Children and adolescents (10 to 17 years of age) :
Quetiapine is not recommended for use in children and adolescents below 18 years of age, due to a lack of data to support use in this age group. Clinical trials with quetiapine have shown that in addition to the known safety profile identified in adults, certain adverse events occurred at a higher frequency in children and adolescents compared to adults (increased appetite, elevations in serum prolactin, and extrapyramidal symptoms) and one was identified that has not been previously seen in adult studies (increases in blood pressure). Changes in thyroid function tests have also been observed in children and adolescents. Furthermore, the long-term safety implications of treatment with quetiapine on growth and maturation have not been studied beyond 26 weeks. Long-term implications for cognitive and behavioural development are not known. In placebo-controlled clinical trials with children and adolescent patients treated with quetiapine, quetiapine was associated with an increased incidence of extrapyramidal symptoms (EPS) compared to placebo in patients treated for schizophrenia and bipolar mania.
Suicide/suicidal thoughts or clinical worsening :
Depression is associated with an increased risk of suicidal thoughts, self-harm and suicide (suicide-related events). This risk persists until significant remission occurs. As improvement may not occur during the first few weeks or more of treatment, patients should be closely monitored until such improvement occurs. It is general clinical experience that the risk of suicide may increase in the early stages of recovery. In addition, physicians should consider the potential risk of suicide-related events after abrupt cessation of quetiapine
treatment, due to the known risk factors for the disease being treated. Other psychiatric conditions for which quetiapine is prescribed, can also be associated with an increased risk of suicide related events. In addition, these conditions may be co-morbid with major depressive episodes. The same precautions observed when treating patients with major depressive episodes should therefore be observed when treating patients with other psychiatric disorders.
Patients with a history of suicide related events, or those exhibiting a significant degree of suicidal ideation prior to commencement of treatment are known to be at greater risk of suicidal thoughts or suicide attempts, and should receive careful monitoring during treatment. A metaanalysis of placebo controlled clinical trials of antidepressant drugs in adult patients with psychiatric disorders showed an increased risk of suicidal behaviour with antidepressants compared to placebo in patients less than 25 years old. Close supervision of patients and in particular those at high risk should accompany drug therapy especially in early treatment and following dose changes. Patients (and caregivers of patients) should be alerted about the need to monitor for any clinical worsening, suicidal behaviour or thoughts and unusual changes in behaviour and to seek medical advice immediately if these symptoms present.
In shorter-term placebo controlled clinical studies of patients with major depressive episodes in bipolar disorder an increased risk of suicide-related events was observed in young adults patients (younger than 25 years of age) who were treated with quetiapine as compared to those treated with placebo (3.0 % vs. 0 %, respectively).
Tardive dyskinesia :
If signs and symptoms of tardive dyskinesia appear, dose reduction or discontinuation of quetiapine should be considered. The symptoms of tardive dyskinesia can worsen or even arise after discontinuation of treatment.
Somnolence and dizziness :
Quetiapine treatment has been associated with somnolence and related symptoms, such as sedation. In clinical trials for treatment of patients with bipolar depression, onset was usually within the first 3 days of treatment and was predominantly of mild to moderate intensity. Bipolar depression patients experiencing somnolence of severe intensity may require more frequent contact for a minimum of 2 weeks from onset of somnolence, or until symptoms improve and treatment discontinuation may need to be considered.
Quetiapine treatment has been associated with orthostatic hypotension and related dizziness which, like somnolence has onset usually during the initial dose-titration period. This could increase the occurrence of accidental injury (fall), especially in the elderly population. Therefore, patients should be advised to exercise caution until they are familiar with the potential effects of the medication.
Cardiovascular :
Quetiapine should be used with caution in patients with known cardiovascular disease, cerebrovascular disease, or other conditions predisposing to hypotension. Quetiapine may induce orthostatic hypotension, especially during the initial dose-titration period and therefore dose reduction or more gradual titration should be considered if this occurs. A slower titration regimen could be considered in patients with underlying cardiovascular disease.
Seizures :
In controlled clinical trials there was no difference in the incidence of seizures in patients treated with quetiapine or placebo. No data is available about the incidence of seizures in patients with a history of seizure disorder. As with other antipsychotics, caution is recommended when treating patients with a history of seizures.
Neuroleptic malignant syndrome :
Neuroleptic malignant syndrome has been associated with antipsychotic treatment, including quetiapine Clinical manifestations include hyperthermia, altered mental status, muscular rigidity, autonomic instability, and increased creatine phosphokinase. In such an event, quetiapine should be discontinued and appropriate medical treatment given.
Severe neutropenia :
Severe neutropenia (neutrophil count < 0.5 X 109/L) has been uncommonly reported in quetiapine clinical trials. Most cases of severe neutropenia have occurred within a couple of months of starting therapy with quetiapine. There was no apparent dose relationship. During post-marketing experience, resolution of leucopenia and/or neutropenia has followed cessation of therapy with quetiapine. Possible risk factors for neutropenia include pre-existing low white cell count (WBC) and history of drug induced neutropenia. Quetiapine should be discontinued in patients with a neutrophil count < 1.0 X 109/L. Patients should be observed for signs and symptoms of infection and neutrophil counts followed (until they exceed 1.5 X 109/L).
Weight :
Weight gain has been reported in patients who have been treated with quetiapine and should be monitored and managed as clinically appropriate as in accordance with utilized antipsychotic guidelines.
Dysphasia :
Dysphasia has been reported with quetiapine. Quetiapine should be used with caution in patients at risk for aspiration pneumonia.
Venous thromboembolism (VTE) :
Cases of venous thromboembolism (VTE) have been reported with antipsychotic drugs. Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible risk factors for VTE should be identified before and during treatment with quetiapine and preventive measures undertaken.
Pancreatitis :
Pancreatitis has been reported in clinical trials and during the post marketing experience. Among the post marketing reports, while not all cases were confounded by risk factors, many patients had factors which are known to be associated with pancreatitis such as increased triglycerides, gallstones, and alcohol consumption.
Additional information :
Quetiapine data in combination with divalproex or lithium in acute moderate to severe manic episodes is limited however, combination therapy was well tolerated. The data showed an additive effect at week 3.QUETIAPINE FUMARATE TABLETS I.P. should be used cautiously in diabetic patients.
QUETIAPINE FUMARATE TABLETS I.P. contain Quetiapine Fumarate I.P. equivalent to Quetiapine 300 mg.

 Cardiovascular
Cardiovascular