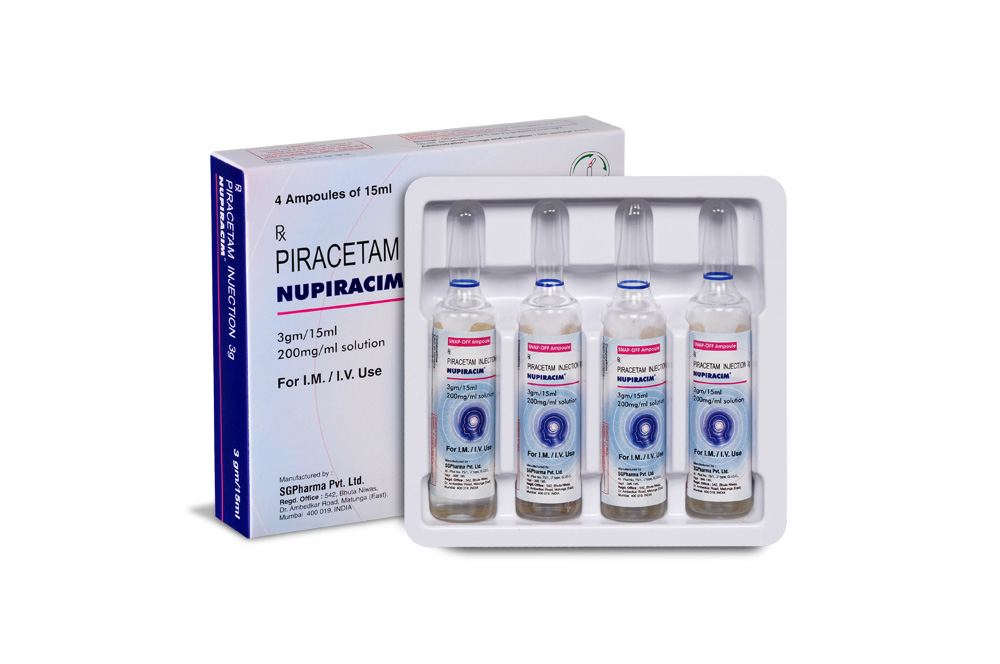
12 gm/60 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
NUPIRACIM (Piracetam) is a nootropic drug with psychotropic action as well as action on the central nervous system. Chemically, Piracetam is 2-(2-oxopyrrolidin-1-yl)acetamide. The molecular formula is C6H10N2O2 and molecular weight is 142.2.
STRUCTURAL FORMULA :
Its structural formula is :
-Strucure.jpg)
NUPIRACIM is a clear, colourless sterile solution.
COMPOSITION :
Each ml contains :
Piracetam B.P. 200 mg
Water for Injection I.P. q.s.
ACTIONS :
Piracetam is a nootropic agent. It improves the metabolic processes in the brain cells, possesses an antihypoxic action, accelerates the reparative processes in functionally damaged cells after traumas, intoxications and stoke. Piracetam acts selectively on the telencephalon, improving its associative functions. The drug has an antiplatelet and a microcirculation ameliorating action. The therapeutic effect of piracetam is manifested after prolonged treatment (several weeks to several months).
PHARMACOKINETICS :
When given by mouth, in either solute or tablet form, piracetam is rapidly and almost totally absorbed in the gut. Bioavailability is almost 100 %. When given as a single 2 g dose, the peak blood level of 40 - 60 mcg/mL is attained after 30 min. In the cerebrospinal fluid, the peak concentration is achieved at 2 - 8 hrs post-dosage. The apparent volume of distribution is in the region of 0.6 L/kg. Plasma half-life is 4 - 5 hrs, while the half-life in the cerebrospinal fluid is 6 - 8 hrs. The half-life is increased in cases of renal insufficiency. Piracetam does not bind to plasma proteins and is eliminated unchanged principally via the kidney. Renal excretion is almost complete, i.e. > 95 %, after 30 hrs. Renal clearance of piracetam in healthy volunteers is 86 mL/min.
Piracetam is dialysable.
Piracetam diffuses into all types of tissue and crosses both the blood-brain barrier and the placenta, as well as the membranes employed in renal dialysis. Piracetam is concentrated in the cerebral cortex, in the frontal, parietal and occipital lobes, in the cerebellum and in the basal ganglia.
INDICATIONS :
Treatment of the elderly with some degree of cerebral functional impairment, e.g. loss of memory, a lack of concentration or alertness, changes of mood, vertigo, personal negligence and deterioration in behaviour. These symptoms can also provide an early warning of the onset of pathological aging, e.g. Alzheimers disease, an Alzheimer type of senile dementia or the dementia produced by multiple cerebral infarcts. Treatment of symptoms related to chronic alcoholism withdrawal and of chronic alcoholic patients suffering from a psycho-organic brain syndrome. Treatment of coma of vascular, traumatic or toxic origin, as well as cognitive deficit and/or vertigo resulting from head injury. Patients suffering from myoclonus of cortical origin, irrespective of aetiology and should be used in combination with other anti-myoclonic therapies.
Administration :
The drug is administered intramuscularly, as a intravenous drip (only Ampoule 5 ml) or as a slow intravenous infusion (Vial 60 ml). Administration of the drug after 4.00 p.m. should be avoided because of its exciting action and the possibility of disturbing the night sleep of the patient.
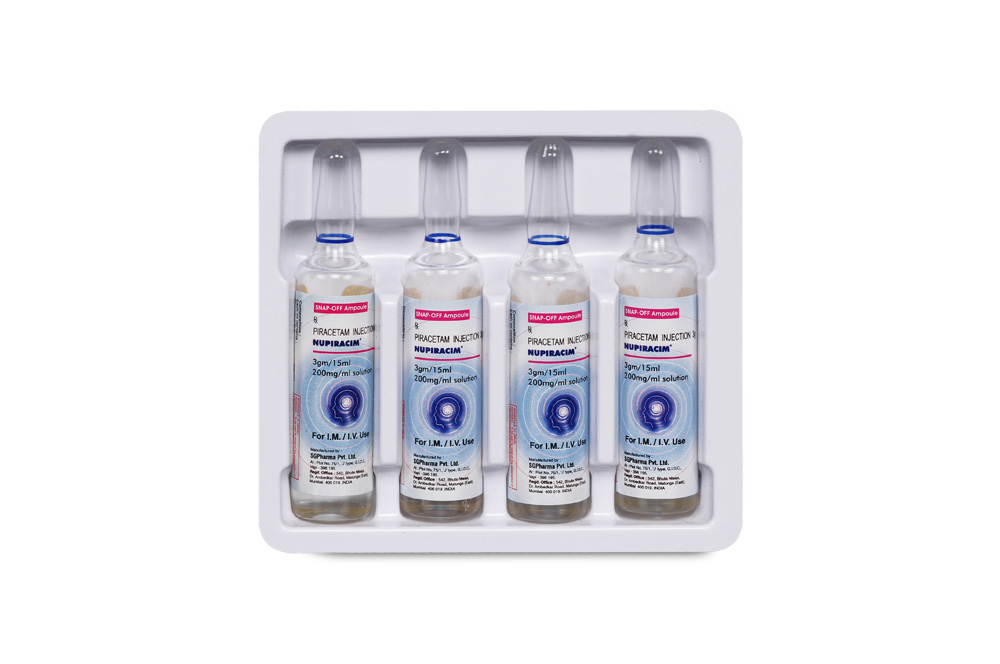
INSTRUCTIONS FOR USE OF AMPOULE (5 ml) :
The ampoule used in this product is equipped with enhanced snap-off opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. Take the ampoule, let the solution at the head of the ampoule to flow
down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the head and gently pressing downwards as shown.

DOSAGE :
The total daily dose can range from 30 - 160 mg/kg/day depending on the indication. This is administered twice daily, but may also be given in 3 or 4 separate doses, either by mouth or parenterally. The oral route is preferred to parenteral if the patients condition permits. Should parenteral treatment be discontinued, the contents of any remaining ampoules can be given by mouth. When treating severe symptoms, 12 g daily may need to be administered as an IV infusion. In the treatment of chronic psycho-organic brain syndrome in the elderly, 4.8 g daily is given initially, followed, after a few weeks, by maintenance therapy at 1.2 - 2.4 g/day.
When treating alcoholism, the dose can be as high as 12 g daily during the initial withdrawal period. Subsequent maintenance therapy is an oral daily dose of 2.4 g. In the treatment of cortical myoclonias, dosage should commence at 7.2 g/day, increasing by 4.8 g/day every 3 or 4 days up to a maximum of 24 g/day, NUPIRACIM being given twice or 3 times daily. Treatment with other anti-myoclonic medicinal products should be maintained at the same dosage. Depending on the clinical benefit obtained, the dosage of other such medicinal products should be reduced, if possible. Once started, treatment with NUPIRACIM should be continued for as long as the original cerebral disease persists. Nevertheless, an attempt should be made every 6 months to decrease or discontinue the medicinal treatment. This should be done by reducing the dose of piracetam by 1.2 g every 2 days, in order to prevent the possibility of sudden relapse.
CONTRAINDICATIONS :
1. Hypersensitivity to piracetam or other pyrrolidone derivatives or any of the excipients.
2. Piracetam is contraindicated in patients with cerebral haemorrhage.
3. Piracetam is contraindicated in end stage renal disease patients or severe parenchymal liver disease.
4. Agitated depression, particularly in the elderly.
WARNINGS :
Treatment with piracetam should not be withdrawn suddenly. The desired synchronisation and enhancement of electric brain activity may lead to an decreased seizure threshold in specially disposed patients (neuronal hyperexcitability). In patients taking antiepileptic drugs the dosage of antiepileptics should be maintained even if the patient reports subjective increase in wellbeing due to the treatment with piracetam.
PRECAUTIONS :
In patients suffering from liver diseases, a monitoring of the liver enzymes and function is necessary. As the principal route of elimination for NUPIRACIM is via the kidney, special care must be taken when treating patients known to suffer from renal insufficiency.
Monitoring of renal function is recommended in such cases. The increase in half-life is directly related to the decrease in renal function and creatinine clearance. This is also true for the older patient in whom creatinine clearance is dependent on age. The daily dose must be individualized according to renal function.
NUPIRACIM should not be prescribed during pregnancy (especially during first trimester) or when breastfeeding, except under exceptional circumstances. NUPIRACIM is able to cross the placenta.
INTERACTIONS :
Piracetam increases the effects of the drugs stimulating the CNS, thyroid hormones and oral anticoagulants. The concurrent administration of neuroleptics increases the risk of hyperkinesia. The classical treatment for alcoholism, i.e. vitamins and sedatives, may continue to be prescribed during cures for alcoholism, in cases where there is a known vitamin deficiency or severe excitability.
SIDE EFFECTS :
Piracetam is well tolerated due to wide therapeutic range. Anxiety, somnolence or insomnia, depression, headache, dizziness, nausea, diarrhoea and hypersensitivity reactions occur rarely. After the administration of high doses nervousness, increased libido and sexual stimulation, hypotrichosis and increased sweating may occur.
OVERDOSAGE and TREATMENT OF OVERDOSAGE :
NUPIRACIM appears to be devoid of toxicity even at very high doses and therefore, the need for specific measures to be taken in case of an overdose is avoided. However, the patients general condition should be closely monitored. Close attention should be given to keep the patient well hydrated and monitor the urine flow. In the case of an overdosage, treatment should be symptomatic.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Unused solutions must be discarded after the dose has been withdrawn.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
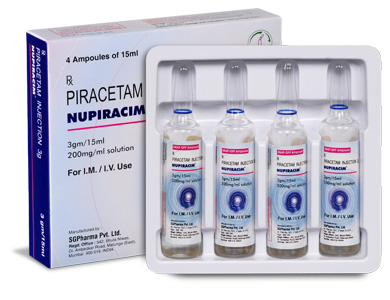
PRESENTATION :
NUPIRACIM is supplied as per below table.
-Table.jpg)
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular