5 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Droperidol injection is a derivative of butyrophenone and has a neuroleptic activity. Chemically Droperidol is 1-1-[3-(p-Fluorobenzoyl)propyl]-1,2,[3,6- tetrahydro -4-pyridyl]-2-benzimidazolinone. Its molecular formula is C22H22FN3O2 and molecular weight is 379.43.
STRUCTURAL FORMULA :
Its structural formula is :
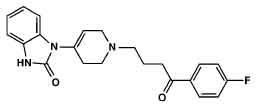
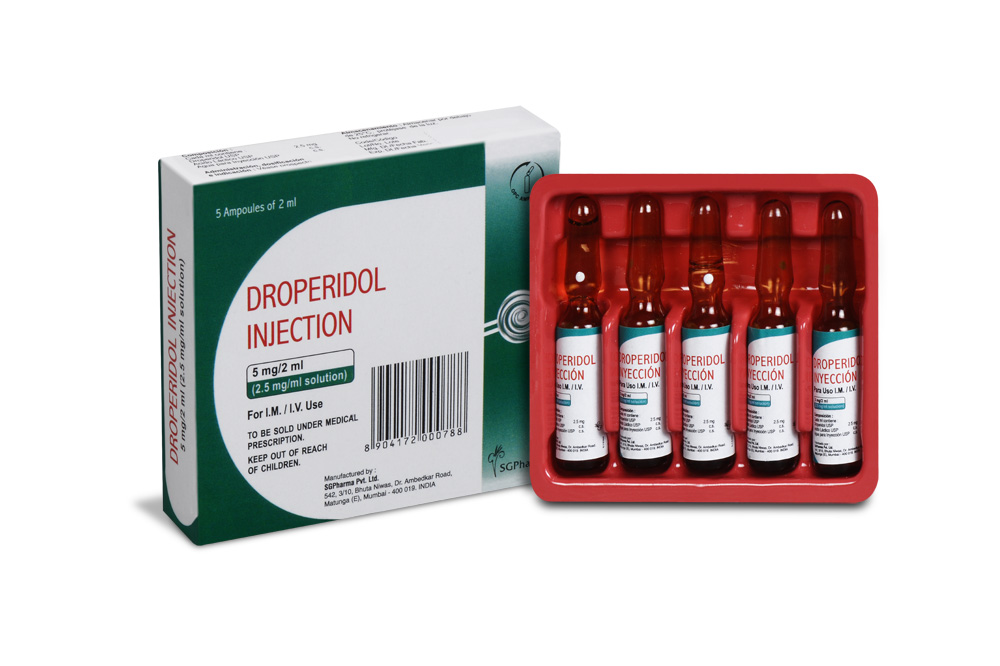
DROPERIDOL INJECTION is a clear, colorless solution filled in ampoule of suitable size.
COMPOSITION :
Each ml contains :
Droperidol USP 2.5 mg
Lactic acid USP q.s.
Water for Injection USP q.s.
ACTIONS :
Droperidol is a butyrophenone neuroleptic agent. Its pharmacological profile is characterised mainly by dopamine-blocking and α1 -adrenolytic effects. Droperidol is devoid of anticholinergic and antihistaminic activity. It has a marked tranquilising and sedative effect, alleviates apprehension and causes a state of mental detachment and indifference while maintaining a state of reflex alertness. Droperidol produces an antiemetic effect. It lowers the incidence of nausea and vomiting during surgical procedures and provides antiemetic protection in postoperative period. Droperidol potentiates other CNS depressants. It induces mild α1- adrenergic blockade and peripheral vascular dilatation and reduces the pressor effect of adrenaline. It can cause hypotension and decreased peripheral vascular resistance and may decrease pulmonary arterial pressure (particularly if it is abnormally high). It may also reduce the incidence of adrenaline induced arrhythmia, but it does not prevent other forms of cardiac arrhythmia.
PHARMACOKINETICS :
The action of a single intramuscular and intravenous dose commences 3 to 10 minutes after administration, although the peak effect may not be apparent for up to 30 minutes. Tranquilising and sedative effects tend to persist for 2 to 4 hours, although alertness may be affected for up to 12 hours. After intravenous administration, plasma concentration fall rapidly during the first 15 minutes. Plasma protein binding is in the range of 85 to 90 %. The distribution volume is 99 to 168 litres. 75 % of the metabolites are eliminated via the kidneys. Only 1 % of the agent is excreted unchanged in urine, and 11 % in faeces. Plasma clearance is 570 mL/min. The elimination half life (t½) is 134 ± 13 minutes. The bioavailability of the oral form is 75 %, the peak concentration being reached after 1 to 2 hours
INDICATIONS :
Droperidol is indicated for the following conditions provided certain precautions are taken : Anaesthesia : Droperidol may be used to produce tranquilisation and to reduce the incidence of nausea and vomiting in surgical and diagnostic procedures. It can also be used for premedication, induction, and as an adjunct in the maintenance of general and regional anaesthesia. In neuroleptanalgesia, Droperidol can be given concurrently with a narcotic analgesic, such as fentanyl injection, to aid in producing tranquillity and in decreasing anxiety and pain. Provided that certain precautions are taken, Droperidol may be administered as a neuroleptic in all types of surgical interventions. The indications of choice for neuroleptanalgesia with Droperidol are major and prolonged surgery, interventions involving high risk for the patient or in aged persons, surgery in patients with a poor overall condition, and in individuals who are in shock. Psychiatry : Droperidol may be used in the management of severe agitation, hyperactivity, or aggressiveness in psychotic disorders, including schizophrenic reaction and the manic type of manic depressive disorder, or in disturbed states, such as some types of acute brain syndrome and in non-psychotic acute excitation states.
Administration :
For Intramuscular and Intravenous use.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
The dosage should be adapted to each individual case. The factors to be considered here include age, body weight, the use of other medications, the type of anaesthesia to be used and the surgical procedure involved. Vital signs should be monitored routinely. To minimise the risk of ventricular arrhythmia, an electrocardiograph (ECG) should be performed and examined for evidence of QT prolongation before any operation commences. ECG monitoring should continue during the surgical procedure and subsequently for a period of time consistent with best medical judgement. This should be at least 7 hours after the end of the procedure. Elderly or debilitated patients or individuals with previously reported adverse reactions to neuroleptic agents may require less Droperidol, and half the normal starting dose may be sufficient for a therapeutic response.
ANAESTHESIA :
Usual adult dosage :
Premedication and diagonostic use : 2.5 mg to 5 mg may be administered intramuscularly or slowly intravenously 30 to 60 minutes before the diagnostic procedure. This (or a lower) dose will reduce the incidence of post-operative nausea and vomiting. The dosage should be reduced as appropriate in the elderly.
Adjuvent to general anaesthesia : Induction : 2.5 mg per 10 kg may be administered (usually intravenously). Smaller dose may be adequate.
Maintenance (if required) : 1.25 to 2.5 mg usually intravenously. An adequate circulation volume should be ensured in view of the alpha-blocking properties of Droperidol.
Adjuvant to regional anaesthesia : 2.5 to 5 mg may be administered intramuscularly or slowly intravenously when additional sedation is required.
Usual paediatric dosage : For children, a reduced dose as low as 1.0 mg per 10 Kg is recommended for premedication or anaethesia induction.
PSYCHIATRY : In psychiatry, the dosage should be determined on an individual basis and is best initiated and titrated under close clinical supervision. To determine the initial dose, the patients age, the symptom severity,and the previous response to other neuroleptic agentsshould be taken into account.
Adults : 5 to 15 mg intravenously or up to 10 mg intramuscularly. The dosage may be repeated at intervals of 4 to 8 hours (intravenous or intramuscular).
Children : 0.5 to 1 mg/day intramuscularly adjusted according to their response.
Elderly : Elderly or debilitated patients or individuals with a history of adverse reactions to neuroleptic agents may require less Droperidol and half the normal starting dose in psychiatry may be sufficient for a therapeutic response. The optimal response in such patients
is usually obtained with more gradual titration and at lower doses. In adolescents, a lower starting dose may be recommended.
CONTRAINDICATIONS :
Droperidol is contraindicated in patients with known hypersensitivity to the agent or its metabolites, in patients with severe depression, in comatose individuals or in patients with Parkinsons disease. Droperidol should not be used in patients with a QTc of greater than
450 msec. Droperidol is contraindicated in patients with acquired long QT interval, such as that associated with concomitant use of medicines known to prolong the QT interval, known hypokalaemia or hypomagnesaemia or clinically significant bradycardia. Droperidol
is also contraindicated in patients with known congenital long QT interval or family history of congenital long QT syndrome.
WARNINGS :
Droperidol should be used in reduced doses in elderly. Caution should be exerted when Droperidol is administered to patients with myasthenia gravis due to its interference with neuromuscular transmission. Administer with caution to patients in whom a sudden drop in blood pressure is undesirable, in phaeochromocytoma and cardiovascular disorders. Care is necessary in epileptic patients. Droperidol may lower the seizure threshold.
PRECAUTION :
Droperidol has a longer duration of action than opioid analgesic e.g. fentanyl, therefore during concurrent use, repeat doses of Droperidol must not be given when only the opioid analgesic is required, since this will lead to accumulation of Droperidol and overdose.
Droperidol is no suitable as an anaesthetic for out patient due to its lethargy duration of action. Other central nervous system depressant used by the patient, whose actions overlap with that of Droperidol, should be reduced in dosage due to their additive or potentiating effect of each other. Care should be exercised when Droperidol is given with other drugs that produce postural hypotension. Dosage adjustments may be necessary. It may also reduce the antihypertensive activity of guanethidine and other adrenergic neurone blockers.
Care should be taken in administration in patients with parkinsonism or diabetes. The effect on the vomiting centre may mask the symptoms of overdose of other agents or of disorders such as gastro-intestinal obstruction.
Pregnancy : Pregnancy Category C
Droperidol is not teratogenic in animals and has been used in a few isolated instances in pregnant women; as with all other pharmacological agents, the benefits of using Droperidol in these situations should be carefully weighed against the possible hazards.
Lactation :
Butyrophenones are excreted in breast milk. If the use of Droperidol is essential, breast-feeding should be avoided.
Use in children :
The safety of Droperidol in children younger than two years of age has not been established. Therefore, this agent is not recommended in this age group.
Use in elderly :
The initial dose of Droperidol should be appropriately reduced in elderly, debilitated and other poor-risk patients. The effect of the initial dose should be considered in determining incremental doses.
INTERACTION :
Medicines known to prolong the QT interval are contraindicated with Droperidol. Examples include certain antiarrhythmics, such as those of Class IA (such as quinidine, disopyramide and procainamide) and Class III (such as amiodarone and sotalol); tricyclic
antidepressants (such as amitriptyline); certain tetracyclic antidepressants (such as maprotiline); certain antipsychotic medications (such as phenothiazines, pimozide and sertindole); certain antihistamines (such as astemizole and terfenadine); cisapride, bepridil, halofantrine and sparfloxacin. Droperidol may potentiate the action of sedative agents (including barbiturates, benzodiazepines, morphinomimetics); the same appliesto antihypertensive agents, whereby orthostatic hypotension mayensue. Like other sedative agents, Droperidol may potentiate respiratory depression caused by opioids. Since Droperidol blocks dopamine receptors, it may inhibit the action of dopamine agonists, such as bromocriptine, lisuride and levodopa. Theoretically, certain agents (e.g. phenobarbitone, carbamazepine, phenytoin), as well as smoking and alcohol consumption, which stimulate metabolising enzymes in the liver, may enhance the metabolic breakdown of neuroleptic agents, possibly necessitating adjustment of the dose.
SIDE EFFECTS :
CNS Effects :
Droperidol may produce Parkinsonian or dyskinetic extrapyramidal side effects. These are readily and completely reversible by treatment with an anti-Parkinsonian agent of the anticholinergic type. In rare cases, paradoxical reactions, including hallucinations, restlessness and isolated cases of anxiety have been observed.
Neuroleptic Malignant Syndrome :
Like other neuroleptic agents, Droperidol has been associated with rare cases of the neuroleptic malignant syndrome, a rarely occurring idiosyncratic response characterised by hyperthermia, generalised muscle rigidity, autonomic instability, and altered consciousness. Hyperthermia is often an early warning sign of this syndrome. In such cases, Droperidol treatment should be discontinued immediately and appropriate supportive therapy and careful monitoring should be initiated.
Tardive Dyskinesia :
As with other neuroleptic agents, tardive dyskinesia may appear in some patients on long-term therapy or after discontinuation of treatment. The syndrome is mainly characterised by involuntary rhythmical movements of the tongue, face, mouth or jaw. The symptoms may persist in some patients. The syndrome may be masked when treatment is reinstituted, when the dosage is increased or when a switch is made to a different antipsychotic agent. Treatment should be discontinued as soon as possible.
Cardiovascular Effects :
Mild to moderate hypotension and occasionally (reflex) tachycardia have been observed following administration of Droperidol. Cases of QT interval prolongation, ventricular arrhythmias and sudden death have been reported rarely. They may occur more frequently with high doses and in predisposed patients.
Endocrine Effects :
Hormonal effects of antipsychotic neuroleptic agents include hyperprolactinaemia which may cause galactorrhoea, gynaecomastia and oligo-or amenorrhoea. Very rare cases of syndrome of Inappropriate ADH Secretion have been reported.
Miscellaneous :
In rare cases, body temperature dysregulation and hypersensitivity reactions such as rash or angio-oedema have been reported.
PHARMACEUTICAL PRECAUTION :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 25°C, protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
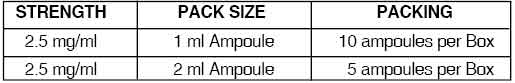
PRESENTATION :
DROPERIDOL INJECTION is supplied as below :
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular