0.500 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Alprostadil is also known as prostaglandin E1. Chemically, Alprostadil is (11α,13E,15S)-11,15 dihydroxy-9-oxo-prost-13-en-1-oic-acid. Its molecular formula is C20H34O5 and its molecular weight is 354.48.
STRUCTURAL FORMULA :
Its structural formula is :
-structure.jpg)
PROSTASOL is a clear colourless solution filled in vial of suitable size.
COMPOSITION :
Each ml contains :
Alprostadil USP 0.500 mg
Absolute Alcohol I.P. q.s.
Contains no preservatives.
ACTIONS :
Alprostadil (prostaglandin E1) is one of a family of naturally occurring acidic lipids with various pharmacologic effects. Vasodilation, inhibition of platelet aggregation, and stimulation of intestinal and uterine smooth muscle are among the most notable of these effects. Intravenous doses of 1 to 10 mcg of Alprostadil per kg of body weight lower the blood pressure in mammals by decreasing peripheral resistance. Reflex increases in cardiac output and rate accompany the reduction in blood pressure. Smooth muscle of the ductus arteriosus is especially sensitive to Alprostadil and strips of lamb ductus markedly relax in the presence of Alprostadil. In addition, administration of Alprostadil reopened the closing ductus of new-born rats, rabbits, and lambs. These observations led to the investigation of Alprostadil in infants who had congenital defects which restricted the pulmonary or systemic blood flow and who depended on a patent ductus arteriosus for adequate blood oxygenation and lower body perfusion.
INDICATIONS :
PROSTASOL is indicated for palliative, not definitive, therapy to temporarily maintain the patency of the ductus arteriosus until corrective or palliative surgery can be performed in neonates who have congenital heart defects and who depend upon the patent ductus for survival. Such congenital heart defects include pulmonary atresia, pulmonary stenosis, tricuspid atresia, tetralogy of Fallot, interruption of the aortic arch, coarctation of the aorta, or transposition of the great vessels with or without other defects.
The preferred route of administration for PROSTASOL is continuous intravenous infusion into a large vein. Alternatively, PROSTASOL may be administered through an umbilical artery catheter placed at the ductal opening. Increases in blood pO2 (torr) have been the same in neonates who received the drug by either route of administration.
Begin infusion with 0.05 to 0.1 mcg Alprostadil per kg of body weight per minute. A starting dose of 0.1 mcg per kg of body weight per minute is the recommended starting dose based on clinical studies; however, adequate clinical response has been reported using a starting dose of 0.05 mcg per kg of body weight per minute. After a therapeutic response is achieved (increased pO2 in infants with restricted pulmonary blood flow or increased systemic blood pressure and blood pH in infants with restricted systemic blood flow), reduce the infusion rate to provide the lowest possible dosage that maintains the response. This may be accomplished by reducing the dosage from 0.1 to 0.05 to 0.025 to 0.01 mcg per kg of body weight per minute. If response to 0.05 mcg per kg of body weight per minute is inadequate, dosage can be increased up to 0.4 mcg per kg of body weight per minute although, in general, higher infusion rates do not produce greater effects.
Dilution Instructions :
To prepare infusion solutions, dilute 1 ml of PROSTASOL with Sodium Chloride Injection I.P. or Dextrose Injection I.P. Undiluted PROSTASOL may interact with the plastic sidewalls of volumetric infusion chambers causing a change in the appearance of the chamber and creating a hazy solution. Should this occur, the solution and the volumetric infusion chamber should be replaced.
When using a volumetric infusion chamber, the appropriate amount of intravenous infusion solution should be added to the chamber first. The undiluted PROSTASOL solution should then be added to the intravenous infusion solution, avoiding direct contact of the undiluted solution with the walls of the volumetric infusion chamber.
Dilute to volumes appropriate for the pump delivery system available. Prepare fresh infusion solutions every 24 hours. Discard any solution more than 24 hours old.
Sample Dilutions and Infusion Rates to Provide a Dosage of 0.1 mcg/kg of Body Weight per Minute
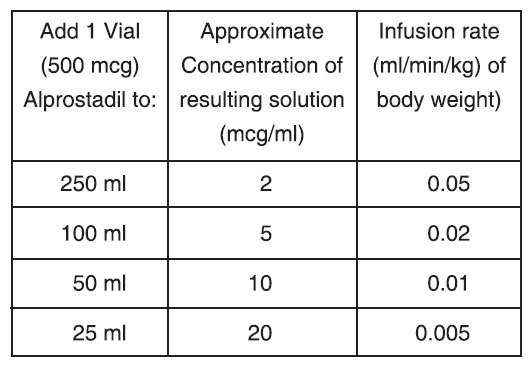
None
NOTE : PROSTASOL must be diluted before it is administered.
The administration of PROSTASOL to neonates may result in gastric outlet obstruction secondary to antral hyperplasia. This effect appears to be related to duration of therapy and cumulative dose of the drug. Neonates receiving PROSTASOL at recommended doses for more than 120 hours should be closely monitored for evidence of antral hyperplasia and gastric outlet obstruction.
PROSTASOL should be infused for the shortest time and at the lowest dose that will produce the desired effects.The risks of long-term infusion of PROSTASOL should be weighed against the possible benefits that critically ill infants may derive from its administration.
PRECAUTIONS :
Apnoea is experienced by about 10 to 12 % of neonates with congenital heart defects treated with Alprostadil Injection. Apnoea is most often seen in neonates weighing less than 2 kg at birth and usually appears during the first hour of drug infusion. Therefore, respiratory status should be monitored throughout treatment and Alprostadil Injection should be used where ventilatory assistance is immediately available.
Pathologic studies of the ductus arteriosis and pulmonary arteries of infants treated with prostaglandin E1 have disclosed histologic changes compatible with a weakening effect upon these structures. The specificity or clinical relevance of these findings is not known. Cortical proliferation of the long bones, has been reported in dogs and neonates during long-term infusions of Alprostadil. The cortical proliferation in infants regressed after withdrawal of the drug. Because Alprostadil inhibits platelet aggregation, use PROSTASOL cautiously in neonates with bleeding tendencies.
PROSTASOL should not be used in neonates with respiratory distress syndrome (hyaline membrane disease). A differential diagnosis should be made between respiratory distress syndrome and cyanotic heart disease (restricted pulmonary blood flow). If full diagnostic facilities are not immediately available, the diagnosis should be based on the presence of cyanosis (pO2 less than 40 torr) and x-ray evidence of restricted pulmonary blood flow.
Necessary Monitoring :
In all neonates, arterial pressure should be monitored intermittently by umbilical artery catheter, auscultation, or with a Doppler transducer. Should arterial pressure fall significantly, decrease the rate of infusion immediately. In infants with restricted pulmonary blood flow, measure efficacy of PROSTASOL by monitoring improvement in blood oxygenation. In infants with restricted systemic blood flow, measure efficacy by monitoring improvement of systemic blood pressure and blood pH.
Carcinogenicity and fertility :
Long-term carcinogenicity and fertility studies have not been done. The Ames and Alkaline Elution assays reveal no potential for mutagenesis. Alprostadil (PGE1) should be administered only by medically trained personnel in facilities in which paediatric patients can receive or have access to paediatric intensive care.
No drug interactions have been reported between Alprostadil Injection and the therapy standard in neonates with restricted pulmonary or systemic blood flow. Standard therapy includes antibiotics, such as Penicillin and Gentamicin; vasopressors, such as Dopamine and Isoproterenol; Cardiac Glycosides; and diuretics, such as Furosemide.
Central Nervous System :
Apnoea has been reported in about 12 % of the neonates treated. Other common adverse reactions reported have been fever in about 14 % of the patients treated and seizures in about 4 %. The following reactions have been reported in less than 1 % of the patients : cerebral bleeding, hyperextension of the neck, hyperirritability, hypothermia, jitteriness, lethargy and stiffness.
Respiratory System :
Apnoea, bradycardia, pyrexia, hypotension and flushing may be signs of drug overdosage. If Apnoea or bradycardia occurs, discontinue the infusion and provide appropriate medical treatment. Caution should be used in restarting the infusion. If pyrexia or hypotension occurs, reduce the infusion rate until these symptoms subside. Flushing is usually a result of incorrect intraarterial catheter placement and the catheter should be repositioned.
PHARMACEUTICAL PRECAUTION :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F). Do not freeze.
PRESENTATION :
PROSTASOL is supplied as 0.500 mg Alprostadil in 1 ml solution. Such 1 Vial is packed in a Box.

 Cardiovascular
Cardiovascular